Biphenyl derivatives
A kind of technology of compound and solvate, applied in the field of new biphenyl derivatives, can solve undisclosed and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
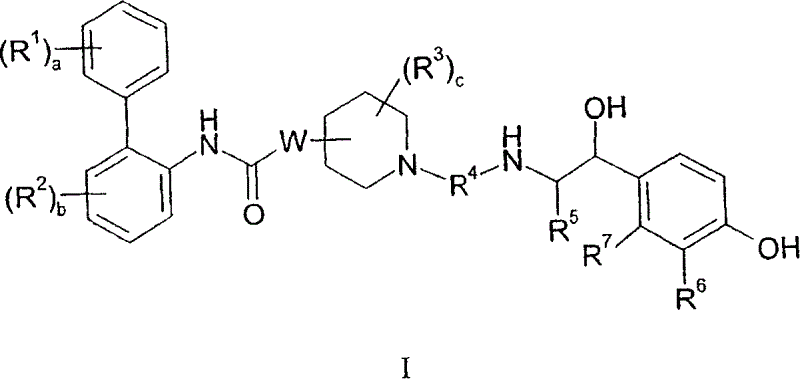
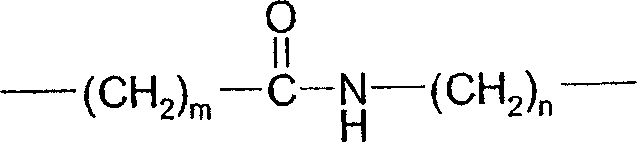
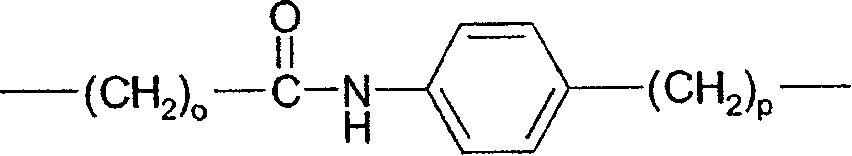
[0081] The following substituents and values are intended to provide representative examples of aspects and embodiments of the invention. These representative values are to further define and explain these aspects and embodiments, and not to eliminate the scope of other embodiments or restricting the scope of the invention. In this regard, the recitation of a preference for a particular value or substituent is not intended in any way to exclude other values or substituents from the invention unless specifically stated otherwise.
[0082] In a particular embodiment of the compound of formula I, a and b are independently 0, 1 or 2; In one embodiment, both a and b are 0.
[0083] When present, R 1 Each can be at the 2, 3, 4, 5 or 6-position of the phenyl ring to which it is attached. In one embodiment, R 1 Each is independently selected from (1-4C)alkyl, halogen, -OR 1a and-NR 1f R 1g ; For example, methyl, fluorine, chlorine, bromine, hydroxyl, methoxy, amino, methy...
preparation Embodiment A
[0562] Dry powders for administration by inhalation are prepared as follows:
[0563] Ingredient Amount
[0564] Compound of the present invention 0.2mg
[0565] Lactose 25mg
[0566] representative method : The compound of the present invention is micronized and mixed with lactose. This blended mixture is filled into gelatin inhalation cartridges. Administer the contents of the cartridge with a powder inhaler.
preparation Embodiment B
[0568] Dry powder formulations for use in dry powder inhalation devices are prepared as follows:
[0569] representative method : Preparation of a pharmaceutical composition in which the ratio of the micronized compound of the present invention to a lactose mixed preparation is 1:200. The composition is filled into a dry powder inhaler device capable of delivering about 10 μg to 100 μg of a compound of the invention per dose.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com