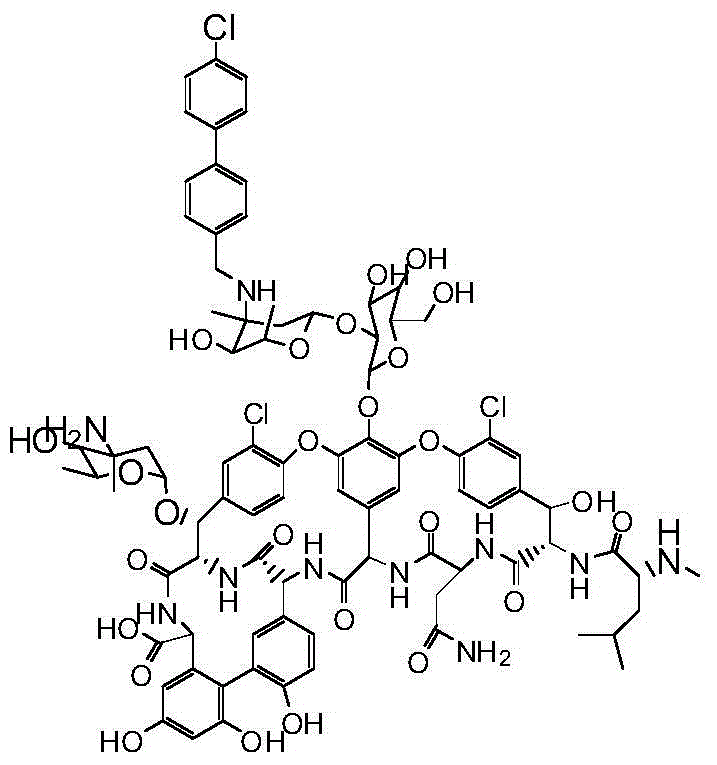
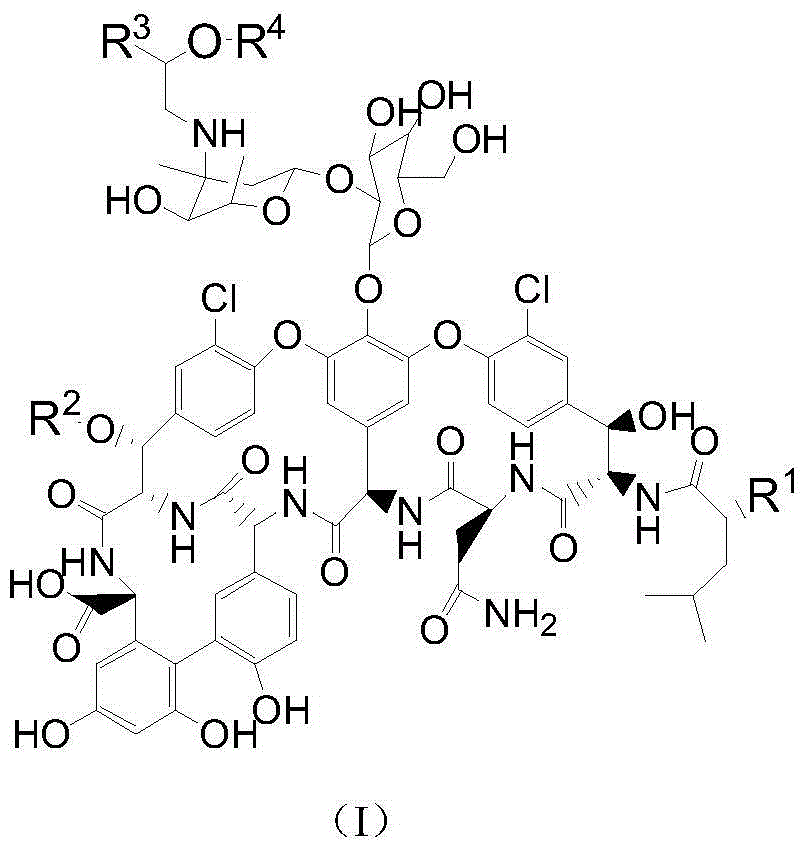
Vancomycin derivative, preparation method and applications thereof
A technology of vancomycin and its derivatives, applied in the field of vancomycin derivatives and its preparation, can solve the problems of increasing the water solubility and fat solubility of compounds, and large fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0241]
[0242] Synthetic steps:
[0243] first step:
[0244]
[0245] Add 2.19g of sodium hydride to a 500ml single-necked bottle, suspend with 100ml of N,N-dimethylformamide, cool to 0-5 degrees under nitrogen protection, dissolve 10.0g of 4-chlorophenylbenzyl alcohol in 100ml of N,N - Dimethylformamide, slowly added dropwise to the reaction solution, after adding and stirring for 0.5 hours, add 7.6 g of methyl bromoacetate dropwise, after adding, raise the temperature to 35-40 degrees and react overnight, after TLC detection, the reaction The solution was poured into 1L of ice water, extracted by adding 500ml of ethyl acetate, the organic phase was washed with saturated sodium chloride, dried over anhydrous sodium sulfate and then spin-dried to obtain the crude product, which was purified by 10% ethyl acetate / petroleum ether column to obtain the oil Liquid 11.0g, yield 83.0%.
[0246] Step two:
[0247]
[0248] Add 2.5g of potassium tert-butoxide into a 100ml ...
Embodiment 2
[0256] Compounds V9, V11, V13, V15, V20, V21, V22, V23, V24, V25, V55, V61 and other compounds can be prepared according to the method in Example 1.
Embodiment 3
[0258]
[0259] Synthetic steps:
[0260] first step:
[0261]
[0262] Add 20ml of n-butanol to a 100ml single-necked bottle, add 1.80g of sodium block in an ice-water bath, heat to reflux until the solid is dissolved, cool to room temperature, add 10.0g of ethyl bromoacetate, after adding, heat up to 40-50 degrees After stirring overnight, after the reaction was detected by TLC, 100 ml of diethyl ether was added, washed with 50 ml of water three times, and the organic phase was spin-dried under reduced pressure to obtain 9.1 g of oily liquid, which could be directly used in the next step.
[0263] Step two:
[0264]
[0265] Add 2.5g of potassium tert-butoxide into a 100ml single-mouth bottle, disperse with 15ml of ether, slowly add 2.2ml of methyl formate solution of 3.0g of the raw material in the previous step under nitrogen protection, react the reaction solution at room temperature overnight, and add ether after the reaction is detected by TLC 50ml, stirred f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com