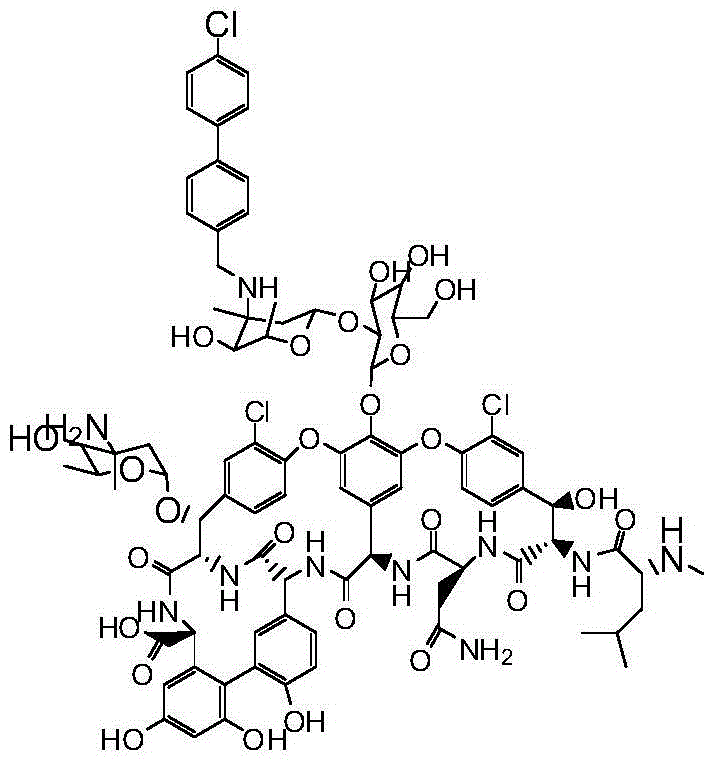
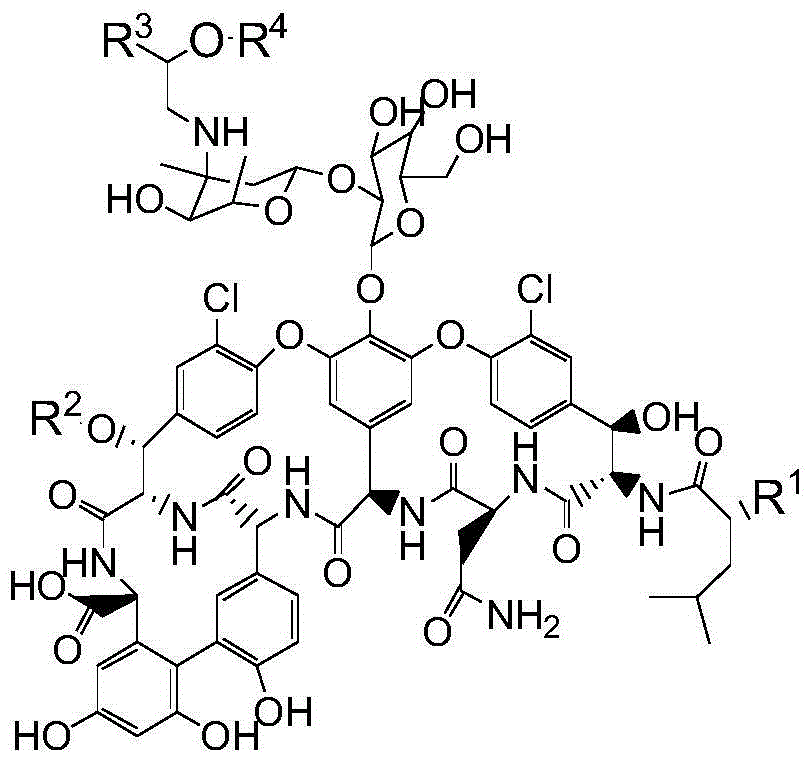
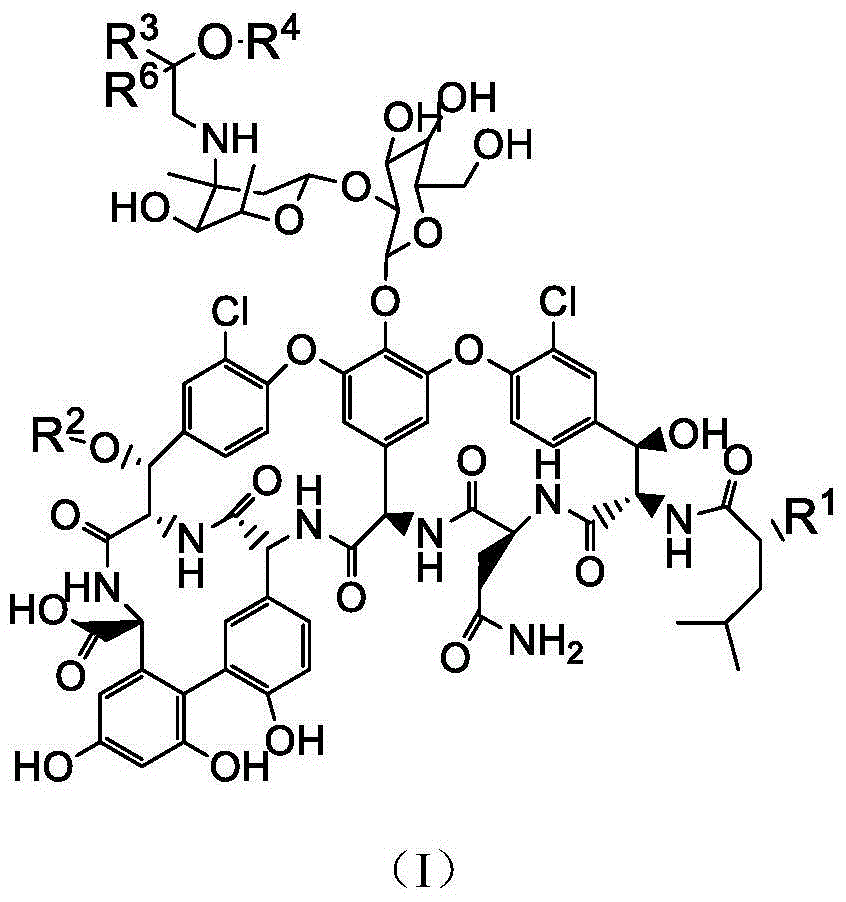
Vancomycin derivatives and preparation and application thereof
A technology of vancomycin and derivatives, applied in the field of vancomycin derivatives, can solve the problems of increasing the water solubility of compounds, reducing the fat solubility, and a single optical isomer.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189]
[0190] 21.6 g of methallyl alcohol, 357 ml of N'N-dimethylformamide and 17 g of imidazole were added to a 1 L single-necked bottle, and after nitrogen replacement, 68.5 g of tert-butyldiphenylchlorosilane was added dropwise. After the dropwise addition was complete, stir overnight. After the reaction was detected by TLC, water and ethyl acetate were added for extraction, the organic phase was dried after liquid separation, and 56.8 g of the product was obtained after being spin-dried and passed through the column, with a yield of 60%.
[0191]
[0192] Into a 250ml three-necked flask, add 7.75g of the product from the previous step, 37g of AD-Mix-α, 25ml of tert-butanol, and 25ml of water, and stir overnight. After the reaction was detected by TLC, water and ethyl acetate were added, the organic phase was dried after liquid separation, and 7.88 g of the product was obtained after being spin-dried and passed through the column, with a yield of 90%.
[0193]
...
Embodiment 2
[0212] Compounds V101, V102, V103, V104, V121, V123, V161, V162, V163, V164, V165, V166 and other compounds can be prepared according to the method in Example 1.
Embodiment 3
[0214]
[0215] Add 1.48g of norvancomycin hydrochloride to a 250ml single-mouth bottle, dissolve it with 80ml of N'N-dimethylformamide, add 332mg of aldehyde, heat and stir at 80 degrees, add 16ml of acetic acid and 126mg of sodium cyanoborohydride, and react After the solution was stirred and reacted for 2 hours, it was cooled to room temperature, 500 ml of diethyl ether was added, filtered, and the filter cake was purified by HPLC to obtain 60.8 g of product V12, with a yield of 48%, MS m / e 1749.5, 1750.5 (M+1).
[0216]
[0217] Add 0.1g of the product from the previous step into a 50ml single-necked bottle, add 1.5ml of tetrahydrofuran, 1.5ml of water, add 24mg of lithium hydroxide monohydrate in batches, add acetic acid to quench after the reaction is completed, spin to dry the solvent, and purify by HPLC to obtain the product V12849mg , 50% yield, MS m / e 1735.5, 1736.5 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com