A crocetin derivative gx-b, its preparation method, and its application in preventing or treating cardiovascular and cerebrovascular diseases
A cardiovascular and cerebrovascular disease, GX-B technology, applied in the direction of cardiovascular system diseases, drug combination, organic chemistry, etc., can solve the problem of poor fat solubility and water solubility of crocetin, clinical application limitations, high drug concentration and dosage To prevent or treat cardiovascular and cerebrovascular diseases, overcome low bioavailability, and improve bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
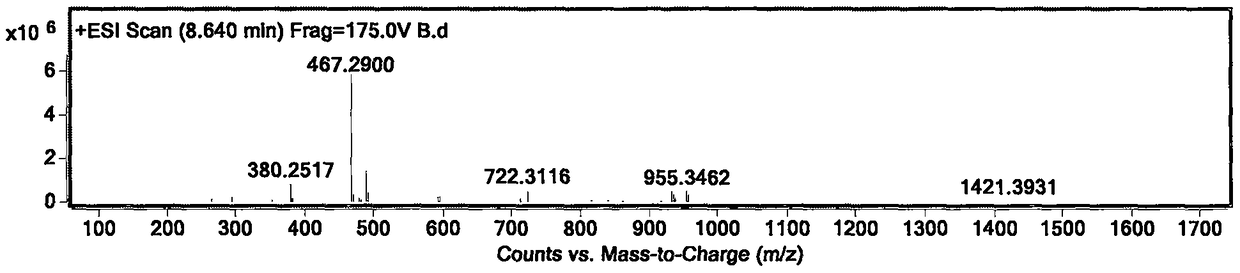
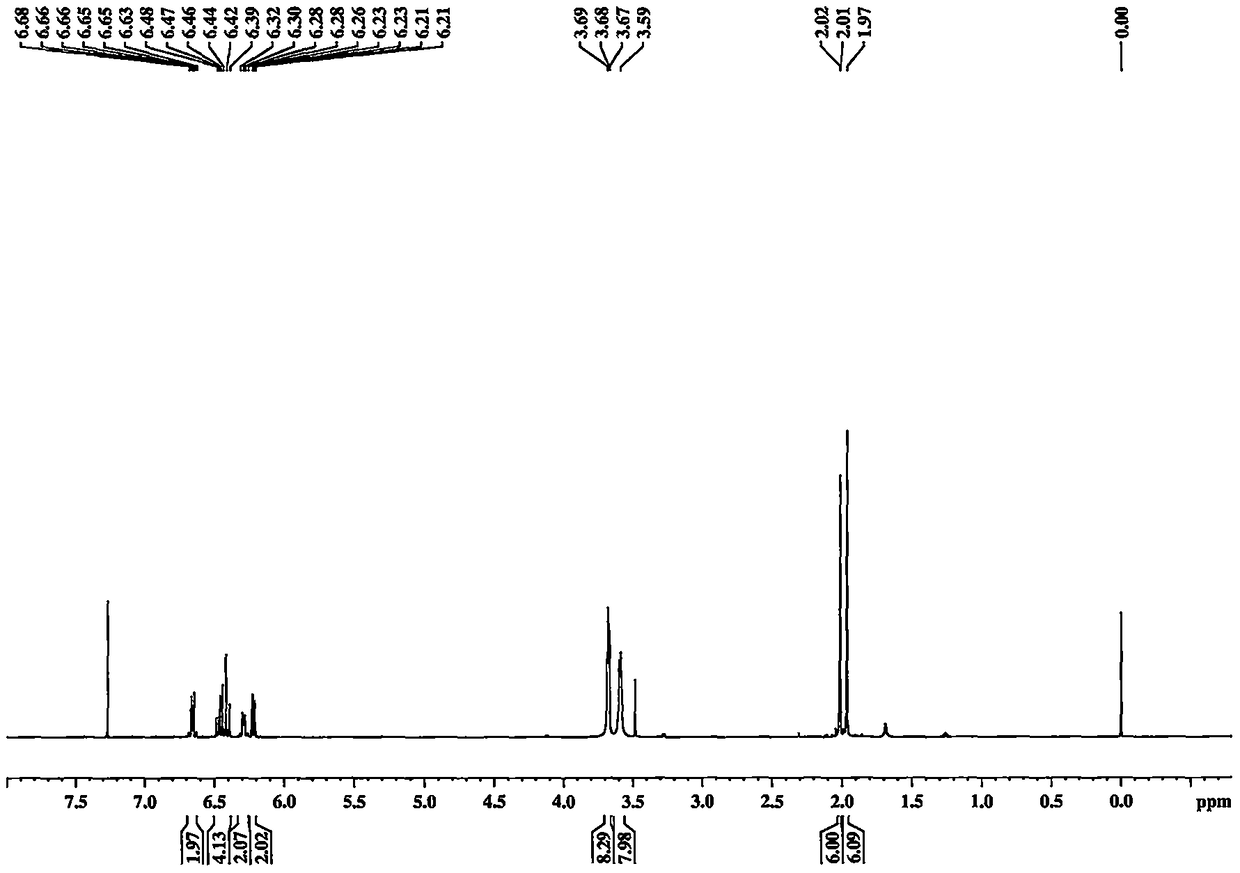
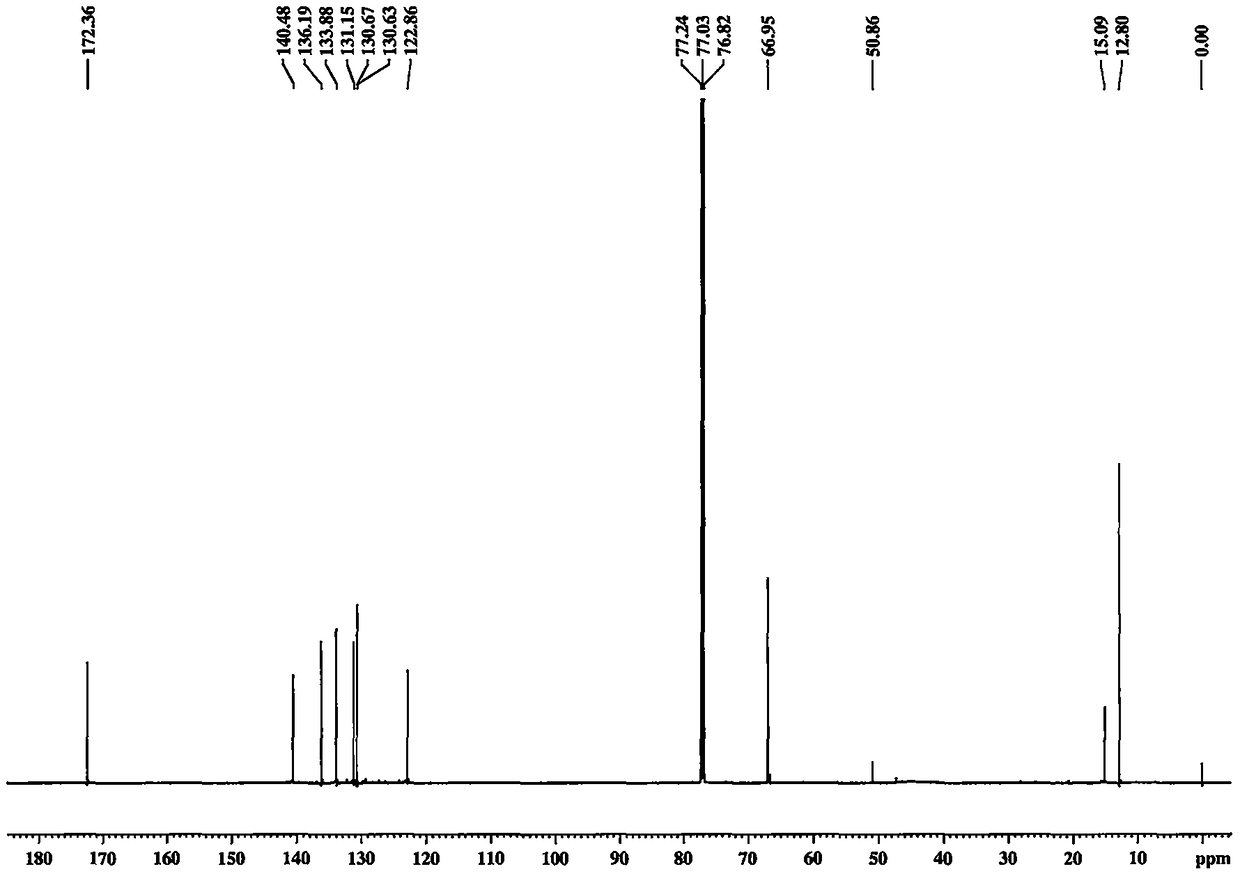
[0040] Embodiment 1 prepares crocetin derivative GX-B
[0041]
[0042] Separately take crocetin GX-1 (purchased from Sigma) (0.5mmol, 164mg), EDCI (1.25mmol, 239mg), HOBt (1.25mmol, 169mg) in 25ml reaction vials. In an ice bath, add Et 3 N (1.25mmol, 172μl) and CH 2 Cl 2 10ml, finally added morpholine (1.0mmol), reacted at 0°C for 4h, then reacted overnight at room temperature. Use TLC and LC-MS to detect whether the product is generated, and stop the reaction after confirming that the reaction is complete. The reaction solution was filtered, the solvent was removed in vacuo, 10ml of EA was added to dissolve, and 2% HCl, 5% NaHCO 3 , H 2 Each 10ml of O was washed 3 times, and finally the solvent EA was removed in vacuo to obtain the crude product. The obtained crude product was separated by a silica gel column, and three column volumes of CHCl 3 Perform elution. The product GX-B was finally obtained, and its structure was characterized by NMR. Yellow powder, yield...
Embodiment 2
[0043] Embodiment 2: Preparation of crocetin derivative GX-M:
[0044]
[0045] Separately take crocetin GX-1 (purchased from Sigma) (0.5mmol, 164mg), EDCI (1.25mmol, 239mg), HOBt (1.25mmol, 169mg) in 25ml reaction vials. Under ice bath condition, add Et3N (2.5mmol, 350μl) and CH 2 Cl 2 20ml, finally added 4-fluorobenzylamine (1.1mmol, 125μl), reacted at 0°C for 4h, then reacted overnight at room temperature. Use TCL and LC-MS to detect whether the product is generated, and stop the reaction after confirming that the reaction is complete. The reaction solution was filtered, the solvent was removed in vacuo, 10ml of EA was added to dissolve, and then washed three times with 2% HCl, 5% NaHCO3, 10ml of H2O respectively, and finally the solvent EA was removed in vacuo to obtain the crude product. The resulting crude product was separated on a silica gel column, eluting with three column volumes of CHCl3. Obtain the product GX-M crude product, then again silica gel column se...
Embodiment 3
[0046] Embodiment 3: Preparation of crocetin derivative GX-N:
[0047]
[0048]Separately take crocetin GX-1 (purchased from Sigma) (0.5mmol, 164mg), EDCI (1.25mmol, 239mg), HOBt (1.25mmol, 169mg) in 25ml reaction vials. Under ice bath condition, add Et3N (2.5mmol, 350μl) and CH 2 Cl 2 20ml, finally added 3,5-difluorobenzylamine (1.1mmol, 130μl), reacted at 0°C for 4h, then reacted overnight at room temperature. Use TCL and LC-MS to detect whether the product is generated, and stop the reaction after confirming that the reaction is complete. The reaction solution was filtered, the solvent was removed in vacuo, 10ml of EA was added to dissolve, and then washed three times with 2% HCl, 5% NaHCO3, 10ml of H2O respectively, and finally the solvent EA was removed in vacuo to obtain the crude product. The resulting crude product was separated on a silica gel column, eluting with three column volumes of CHCl3. The crude product GX-N was obtained, and then separated on a silica...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com