A crocetin derivative gx-ring, its preparation method, and its application in preventing or treating cardiovascular and cerebrovascular diseases
A technology of crocus acid and crocus, applied in the field of new crocus acid derivatives, can solve the problems of poor fat solubility and water solubility of crocus acid, limited clinical application, low bioavailability, etc. Or treat cardiovascular and cerebrovascular diseases, improve bioavailability, and overcome the effects of extremely low fat solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
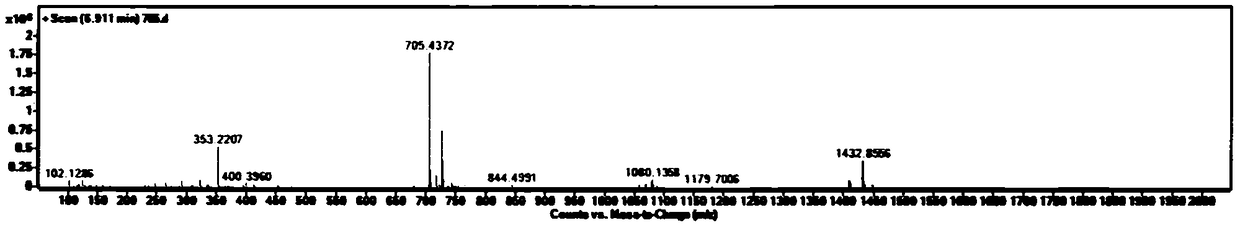
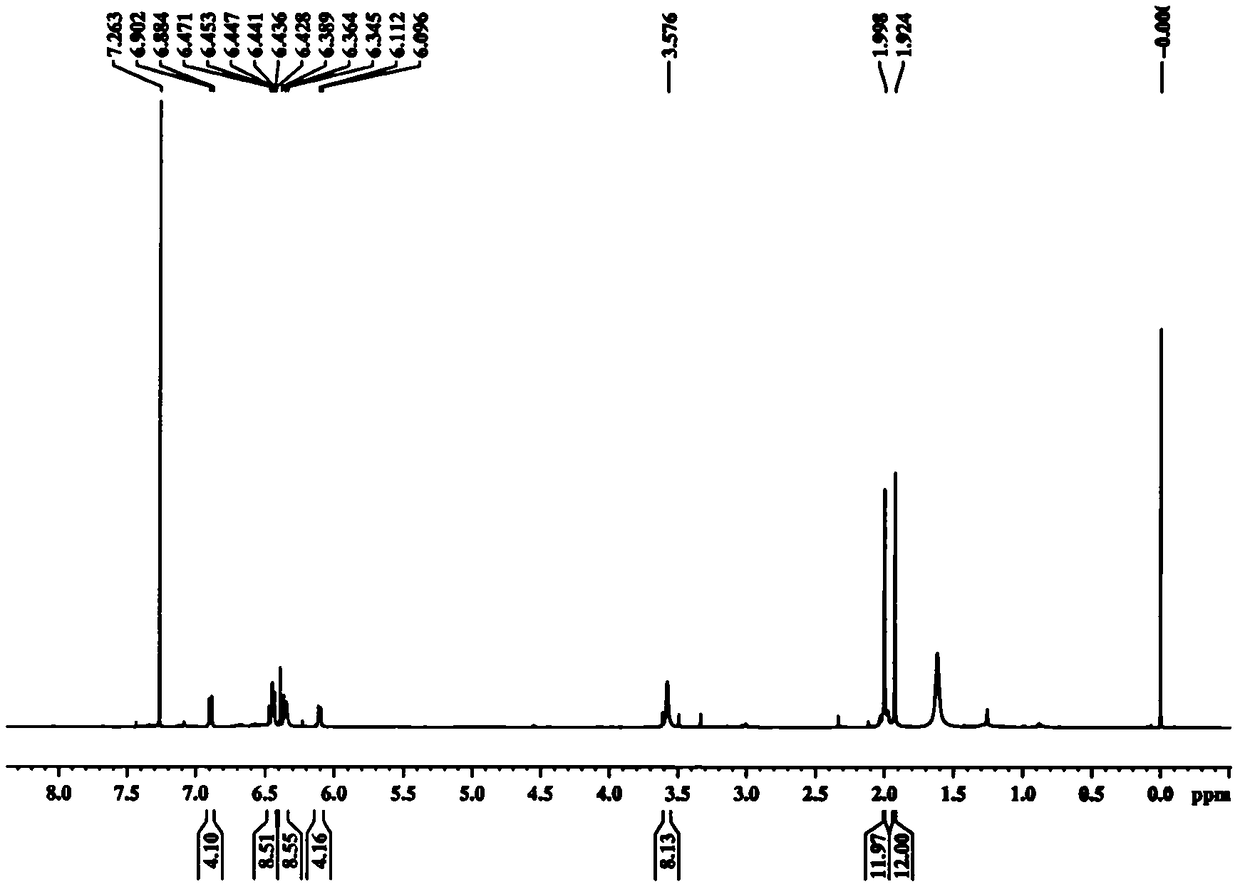
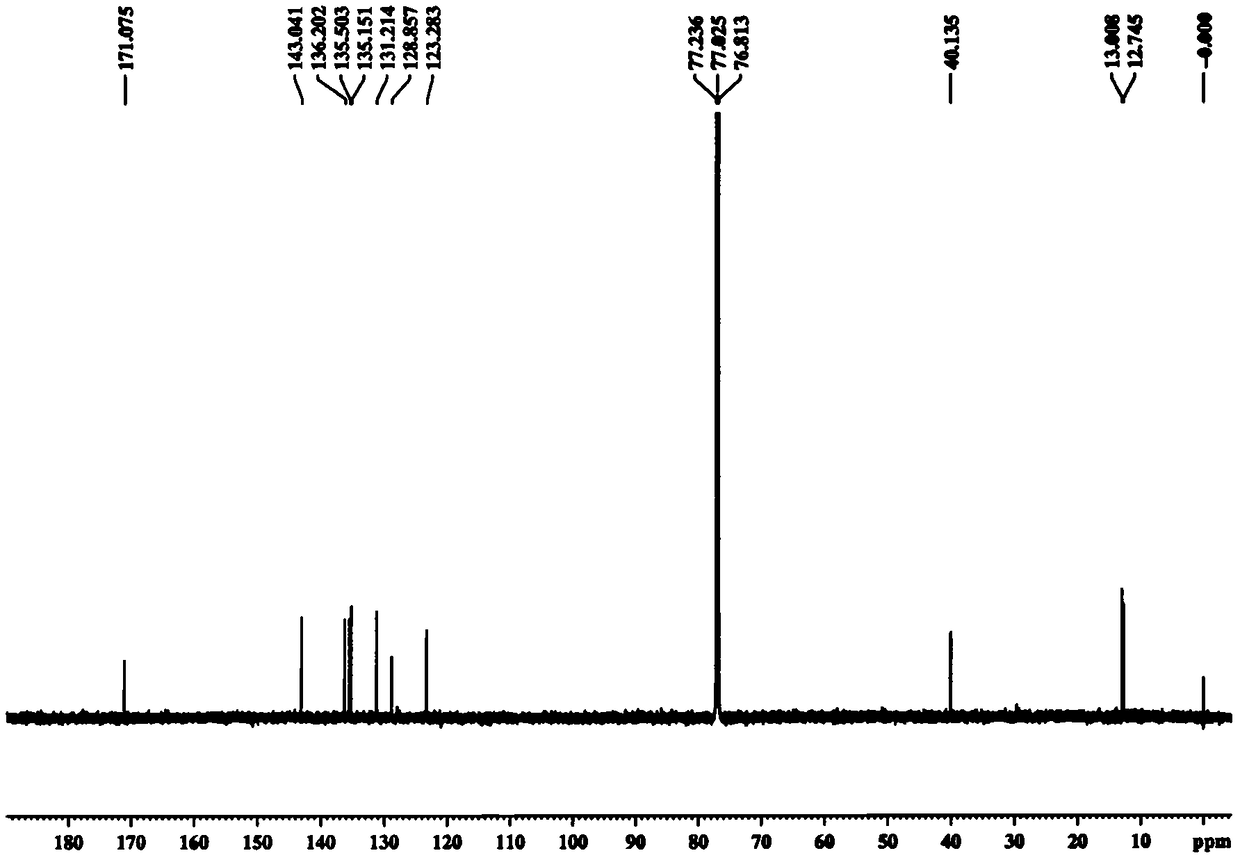
[0036] Embodiment 1 prepares crocetin derivative GX-Ring
[0037]
[0038] Take GX-1 (purchased from Sigma) (0.25mmol, 80mg) in a 100ml reaction bottle. Add 220ml of CH2Cl, add oxalyl chloride (0.1mmol, 100μl) and 1-2 drops of DMF under ice-bath conditions, and stir at room temperature for 4h. When the color of the solution is dark red and basically clear, add ethylenediamine (0.25mmol) and triethylamine (2.0mmol,) to react, react at room temperature overnight, add 100μl H2O and stir the reaction for 5 hours to stop the reaction, use TLC and LC- MS detects whether the product is formed. The reaction solution was filtered, the solvent was removed in vacuo, and 20ml of EA was added to dissolve it, followed by washing with 2% HCl, 5% NaHCO3, and 20ml of H2O three times respectively, and the solvent was removed in vacuo to obtain a crude product. The GX-Ring is separated with a silica gel column, first eluted with 2-3 column volumes of CHCl3, and then the polarity of the elue...
Embodiment 2
[0039] Embodiment 2: Preparation of crocetin derivative GX-M:
[0040]
[0041] Separately take crocetin GX-1 (purchased from Sigma) (0.5mmol, 164mg), EDCI (1.25mmol, 239mg), HOBt (1.25mmol, 169mg) in 25ml reaction vials. Under ice-bath conditions, Et3N (2.5mmol, 350μl) and CH2Cl220ml were added, and finally 4-fluorobenzylamine (1.1mmol, 125μl) was added, reacted at 0°C for 4h, and then reacted overnight at room temperature. Use TCL and LC-MS to detect whether the product is generated, and stop the reaction after confirming that the reaction is complete. The reaction solution was filtered, the solvent was removed in vacuo, 10ml of EA was added to dissolve, and then washed three times with 2% HCl, 5% NaHCO3, 10ml of H2O respectively, and finally the solvent EA was removed in vacuo to obtain the crude product. The resulting crude product was separated on a silica gel column, eluting with three column volumes of CHCl3. Obtain the product GX-M crude product, then again silica...
Embodiment 3
[0042] Embodiment 3: Preparation of crocetin derivative GX-N:
[0043]
[0044]Separately take crocetin GX-1 (purchased from Sigma) (0.5mmol, 164mg), EDCI (1.25mmol, 239mg), HOBt (1.25mmol, 169mg) in 25ml reaction vials. Under ice-bath conditions, Et3N (2.5mmol, 350μl) and CH2Cl220ml were added, and finally 3,5-difluorobenzylamine (1.1mmol, 130μl) was added, reacted at 0°C for 4h, and then reacted overnight at room temperature. Use TCL and LC-MS to detect whether the product is generated, and stop the reaction after confirming that the reaction is complete. The reaction solution was filtered, the solvent was removed in vacuo, 10ml of EA was added to dissolve, and then washed three times with 2% HCl, 5% NaHCO3, 10ml of H2O respectively, and finally the solvent EA was removed in vacuo to obtain the crude product. The resulting crude product was separated on a silica gel column, eluting with three column volumes of CHCl3. The crude product GX-N was obtained, and then separat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com