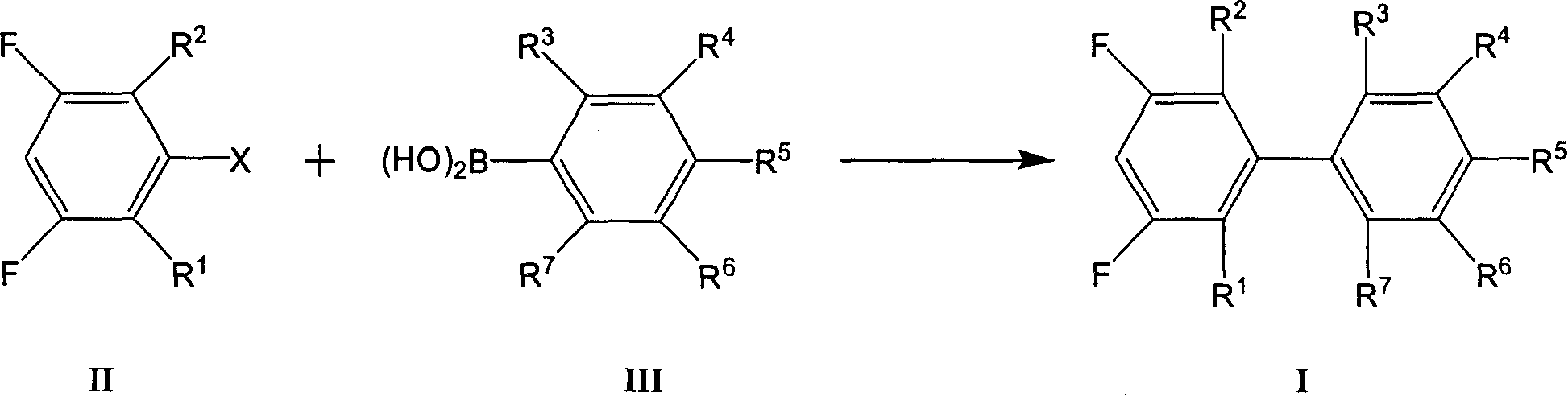
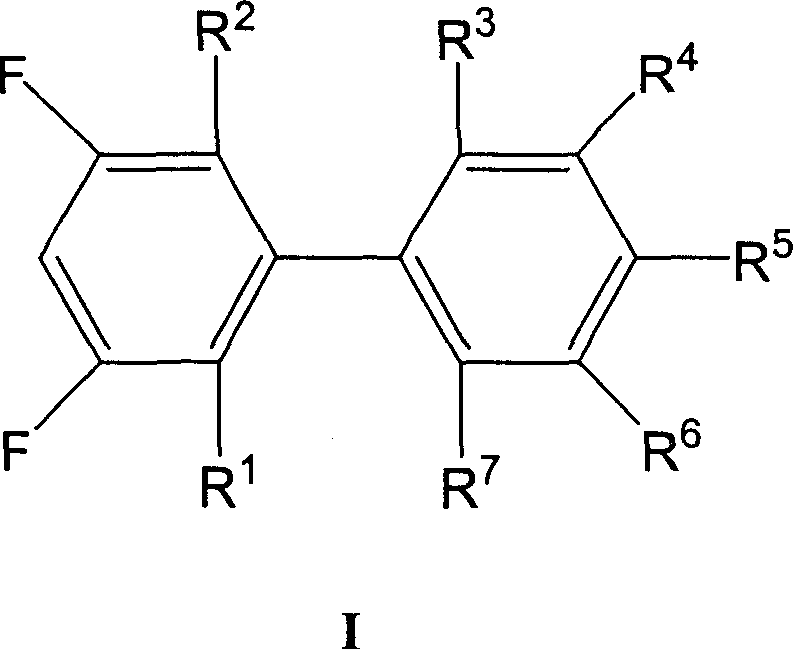
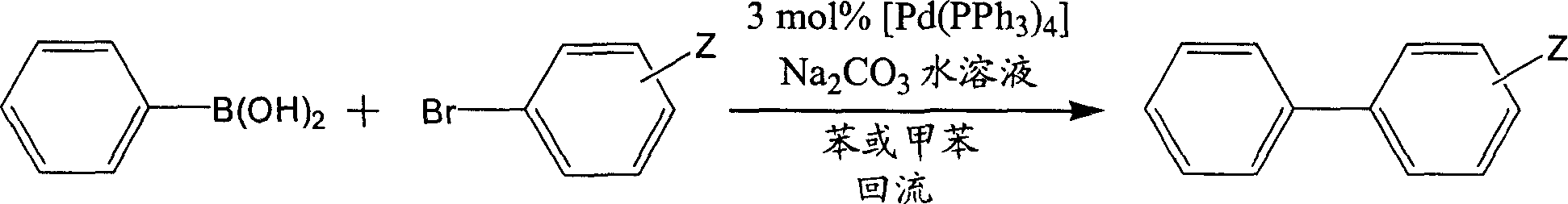Preparation method of 3,5-difluoro biphenyl derivative
A compound and general formula technology, applied in the field of preparation 3, can solve the problems of high cost, large amount of catalyst, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0056] In a four-necked flask equipped with a stirrer, a thermometer, a reflux condenser and a nitrogen inlet, nitrogen replacement was first performed. Then add 4-propylphenylboronic acid 68g (0.42 mole) under nitrogen protection, toluene 427g (4.63 mole), salt of wormwood 105g (0.76 mole), water 269g (14.93 mole), 3,5-difluorobromobenzene 58g ( 0.3 mol) and ethanol 126g (2.74 mol). Add catalyst Pd(PPh 3 ) 4 0.3g (2.6×10 -4mol) and heated to reflux for 10 hours. After cooling to room temperature, the layers were separated, the aqueous layer was extracted with toluene, and the organic layers were combined. After the organic layer was distilled off the solvent under reduced pressure, 55 g of fractions at 128-132° C. / 1 mmHg were intercepted, with a purity of 99.4% and a yield of 78.6%.
[0057] Infrared spectrum (cm -1 ): 3080, 3028, 2960, 2931, 2872, 1907, 1790, 1624, 1595, 1452, 1338, 1116, 989, 862, 839, 682.
[0058] Mass spectrum (m / z): 232, 203, 188, 183, 164, 138, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com