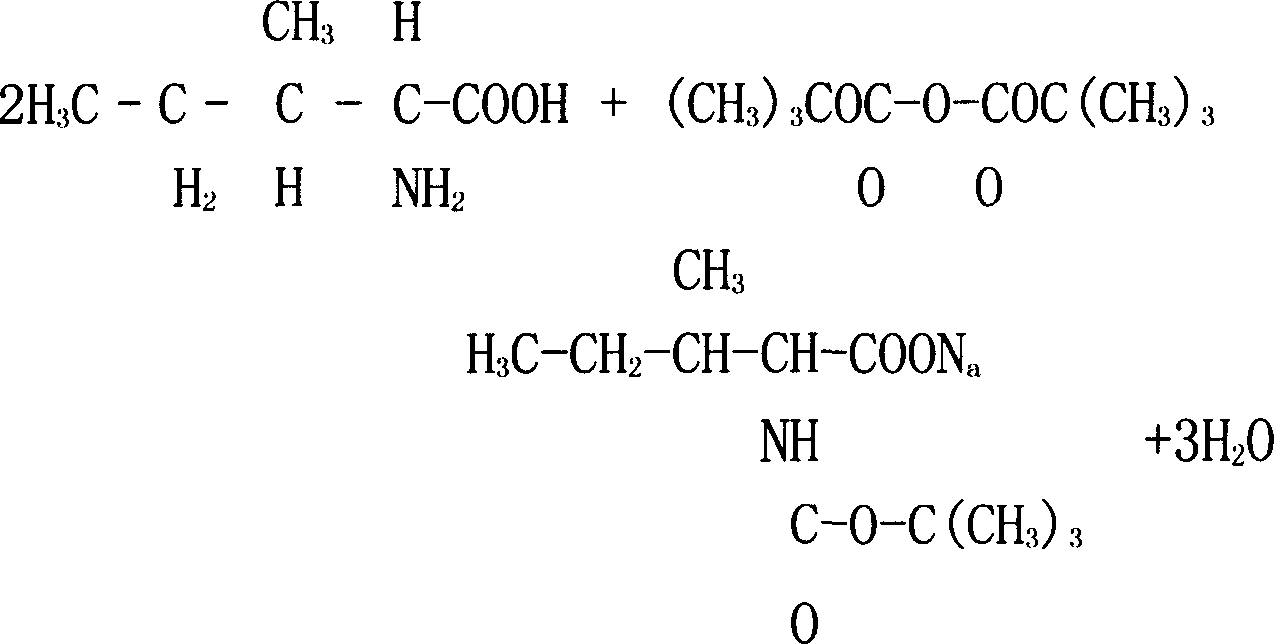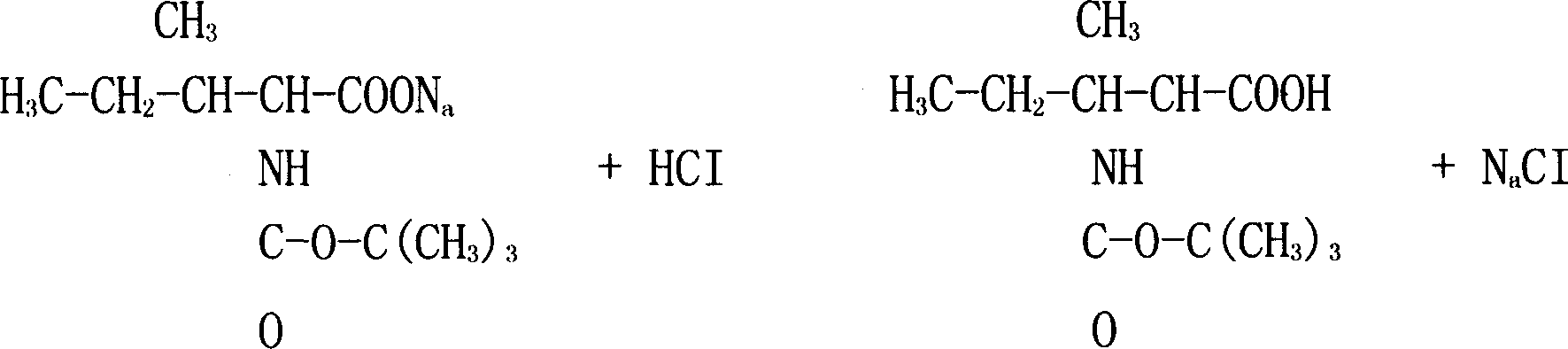Method for synthesizing N-tert-butoxy-oxo-L-isoleucine
A technology of tert-butoxycarbonyl and isoleucine, which is applied in the chemical industry, can solve problems such as high product cost, high price, and long process time, and achieve the effects of reducing production costs, shortening production cycles, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0012] The present invention synthesizes N-tert-butoxycarbonyl-L-isoleucine, the main raw material that adopts is L-isoleucine, BOC acid anhydride and 50% dioxane aqueous solution, and the mol ratio of raw material is 1: 1.66:7.22, the procedure is as follows: 131.18 grams of L-isoleucine, 218 grams of BOC anhydride and 960 grams of 50% dioxane aqueous solution are placed in the reactor and stirred to fully dissolve the L-isoleucine After putting into ice brine to cool, the chemical reaction equation is:
[0013]
[0014] Add 4N sodium hydroxide aqueous solution dropwise for 2 hours to maintain the pH value of the reaction system at 9-10. When the pH value is constant at 9, the reaction is terminated and the temperature is controlled at 15-25°C;
[0015] Extract twice with 900 mL of ethyl acetate, leave a layer of water, add hydrochloric acid to make the pH value of the solution 9, the chemical reaction equation is:
[0016]
[0017] Extract twice with 900 mL of ethyl a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com