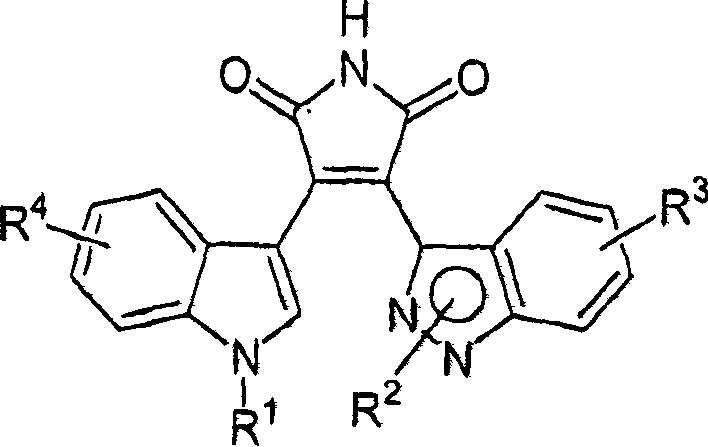
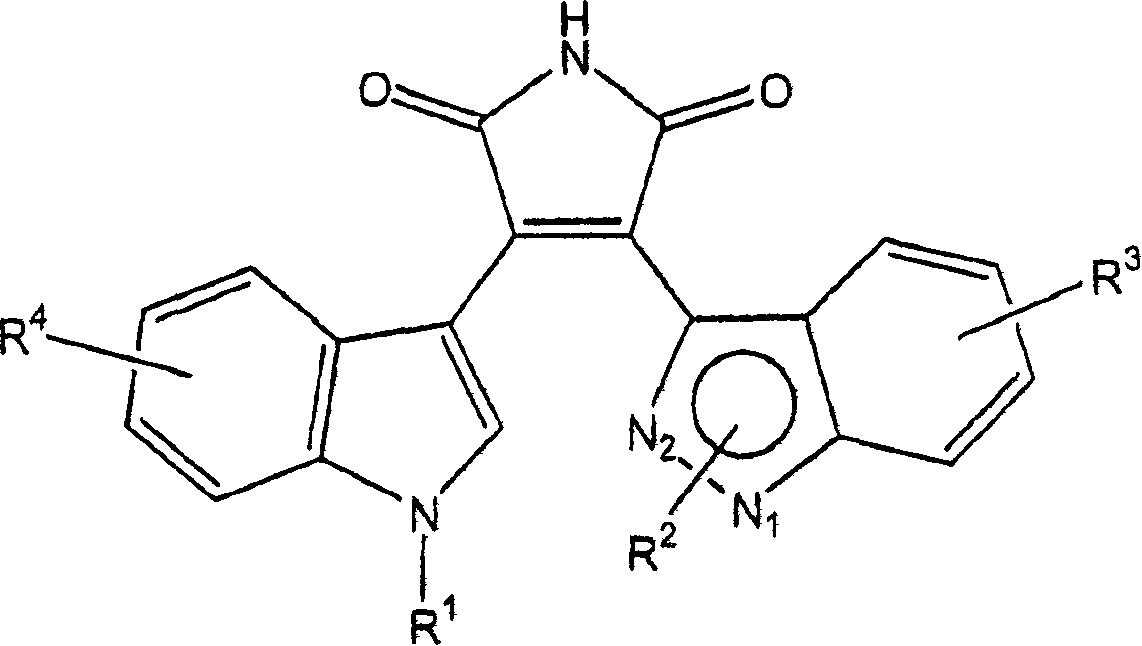
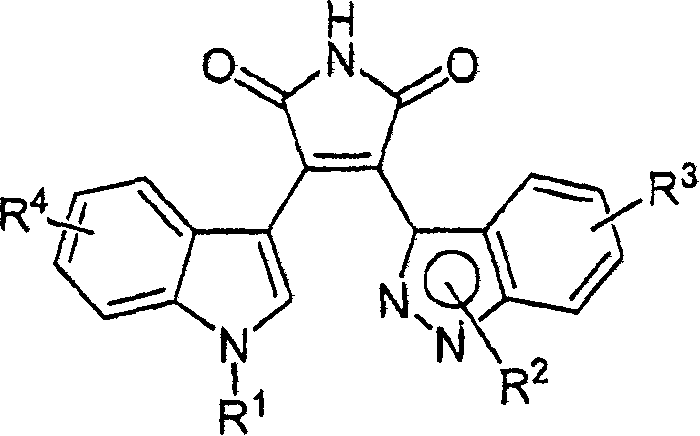
Substituted indazolyl(indolyl)maleimide derivatives as kinase inhibitors
A technology of substituents and alkyl groups, applied in the field of novel compounds, can solve problems such as undisclosed pyrroline compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] 3-(5-Chloro-1-methyl-1H-indol-3-yl)-4-[1-(3-imidazol-1-yl-propyl)-1H-indazol-3-yl]-
[0203] Pyrrole-2,5-dione (Compound 1)
[0204] Pyridine (1.024g, 12.95mmol) and methanesulfonic anhydride (1.3g, 7.45mmol) were added to compound 1a (1.62g, 3.7mmol, prepared see WO 02 / 46183) in THF (5OmL). The mixture was heated at 50°C for 3 hours and then cooled to room temperature. Additional THF (10 mL) was added, followed by 1N HCl (10 mL). The mixture was stirred for 15 minutes, then extracted several times with EtOAc. The combined EtOAc layers were washed once with 1N HCl (10 mL), water (2×20 mL) and saturated NaCl (20 mL), dried (Na 2 SO 4 ) and evaporated in vacuo to obtain compound 1b (1.6 g, 84%) as a reddish solid. ES-MS mz 513 (MH + ).
[0205] 75% NaH (3.74 mg, 0.117 mmol) was added to a mixture of imidazole (7.96 mg, 0.117 mmol) in DMF (5 mL) at 0 °C, and the mixture was heated to reflux for 30 min. The solution was then cooled to room temperatur...
Embodiment 2
[0208] 3-(5-Chloro-1-methyl-1H-indol-3-yl)-4-[1-(3-[1,2,3]triazol-1-yl-propyl)-1H- Indazole-
[0209] 3-yl]-pyrrole-2,5-dione (compound 2)
[0210] 75% NaH (3 mg, 0.093 mmol) was added to a mixture of triazole (5 mg, 0.072 mmol) in DMF (4 mL) at 0°C. The mixture was heated to reflux for 30 minutes. The solution was then cooled to room temperature. Compound 1b (20 mg, 0.03 mmol) in DMF (1 mL) was added dropwise. The mixture was heated to 80 °C for 1 h, then stirred at room temperature overnight. TLC showed some starting material was also contained. The mixture was heated to 90°C overnight. The solvent was evaporated in vacuo to a dark oil. The oil was purified by preparative TLC to obtain compound 2 (3 mg) as an orange solid. 1 HNMR (CDCl 3 )δ8.08(s, 1H), 7.70(d, J=8.2Hz, 1H), 7.64(s, 1H), 7.43(m, 3H), 7.17(m, 2H), 7.01(dd, J=1.9 , 8.7Hz, 1H), 6.12(d, J=1.8Hz, 1H), 4.31(t, J=6.2Hz, 2H), 4.12(t, J=6.5Hz, 2H), 3.86(s, 3H), 2.40 (m, 2H). ES-MS m / z 486 (MH ...
Embodiment 3
[0214] 3-(5-chloro-1-methyl-1H-indol-3-yl)-4-[1-(3-tetrazol-2-yl-propyl)-1H-indazol-3-yl]
[0215] Pyrrole-2,5-dione
[0216] and
[0217] 3-(5-chloro-1-methyl-1H-indol-3-yl)-4-[1-(3-tetrazol-1-yl-propyl)-1H-indazol-3-yl] -
[0218] Pyrrole-2,5-dione (compound 3 and compound 4)
[0219] 3% tetrazole CH 3 CN solution (10.15 mL, 3.4 mmol) was diluted with DMF (10 mL), potassium carbonate (0.47 g, 3.4 mmol) was added, and the mixture was heated to 90° C. for 2 h. Cool the mixture to room temperature. Compound 1b (350 mg, 0.68 mmol) in DMF (5 mL) was added dropwise. The mixture was heated to 80°C overnight. The solvent was evaporated. Water (10 mL) was added and the mixture was extracted with EtOAc (3 x 50 mL). The combined organic layers were washed with H 2 O and brine washed, dried (Na 2 SO 4 ) and evaporated in vacuo to a dark oil. The oil was purified by flash column chromatography (96:4:0.4; DCM:MeOH:NH 4 OH), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com