ErbB surface receptor complexes as biomarkers
A technology of complexes and receptors, applied in biochemical equipment and methods, microbial measurement/inspection, etc., can solve problems such as inability or impractical use, inconvenient measurement technology, lack of sensitivity, etc., to increase costs and workload Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Her-2 heterodimerization on cell lysates
[0156] and analysis of receptor phosphorylation
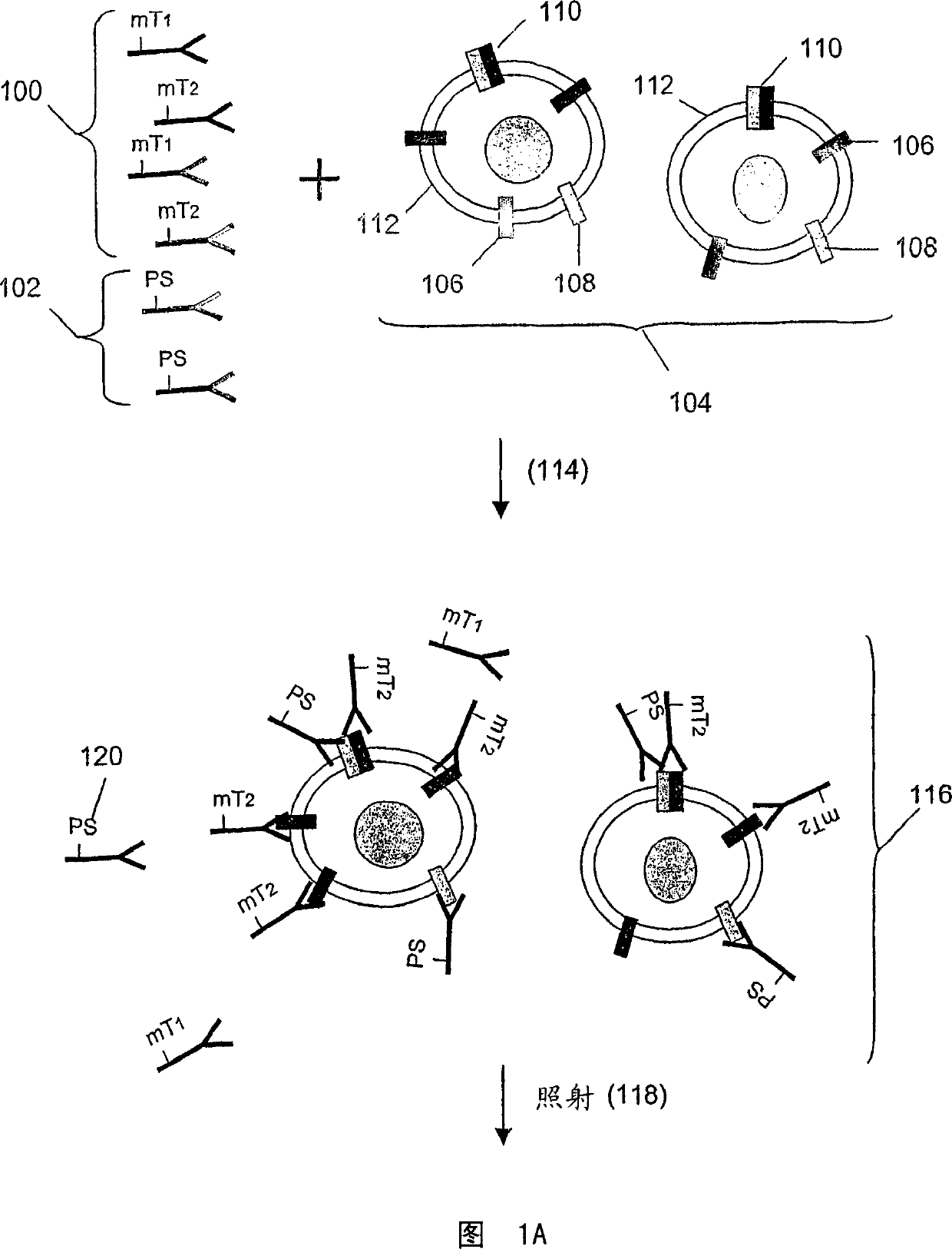
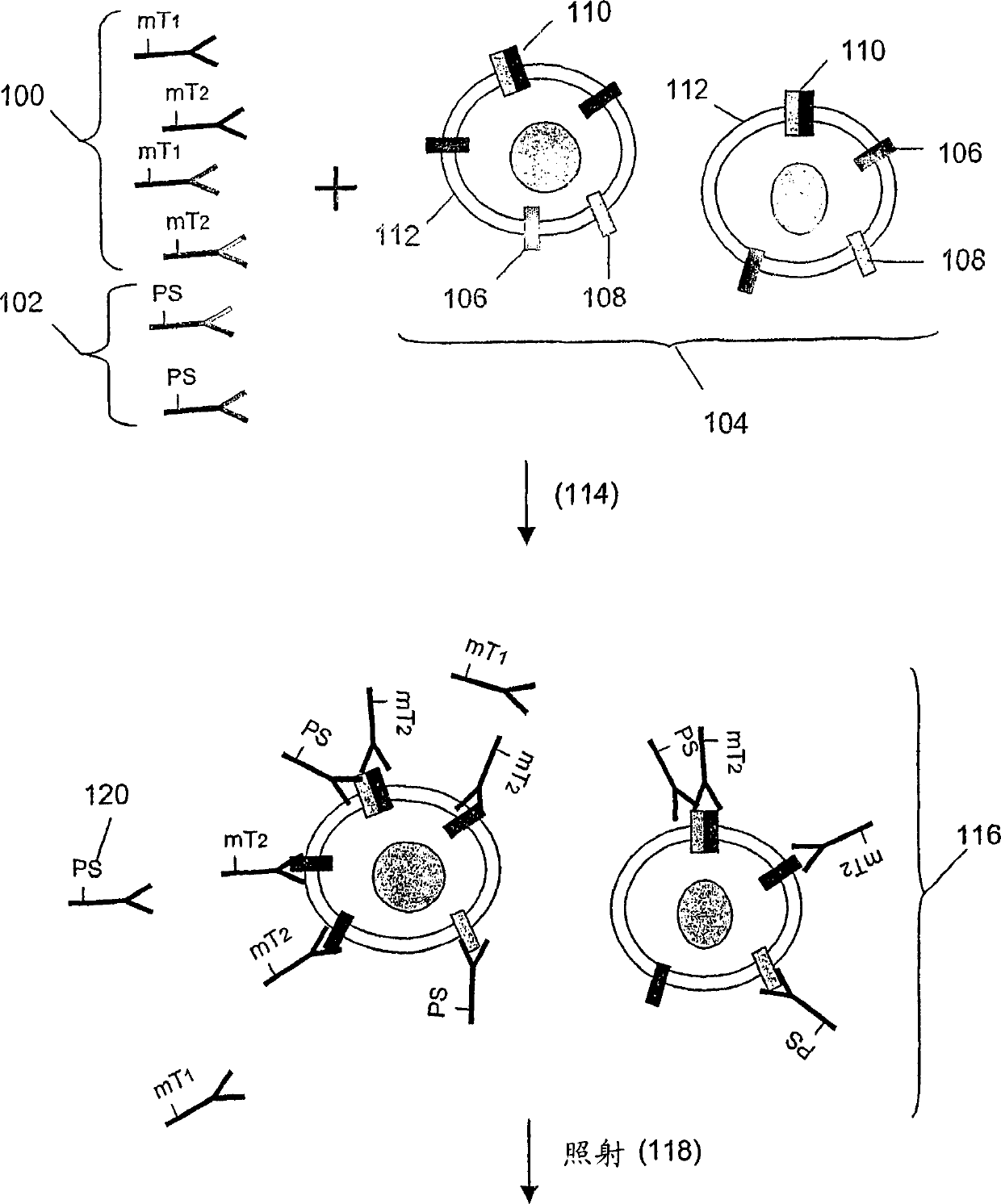
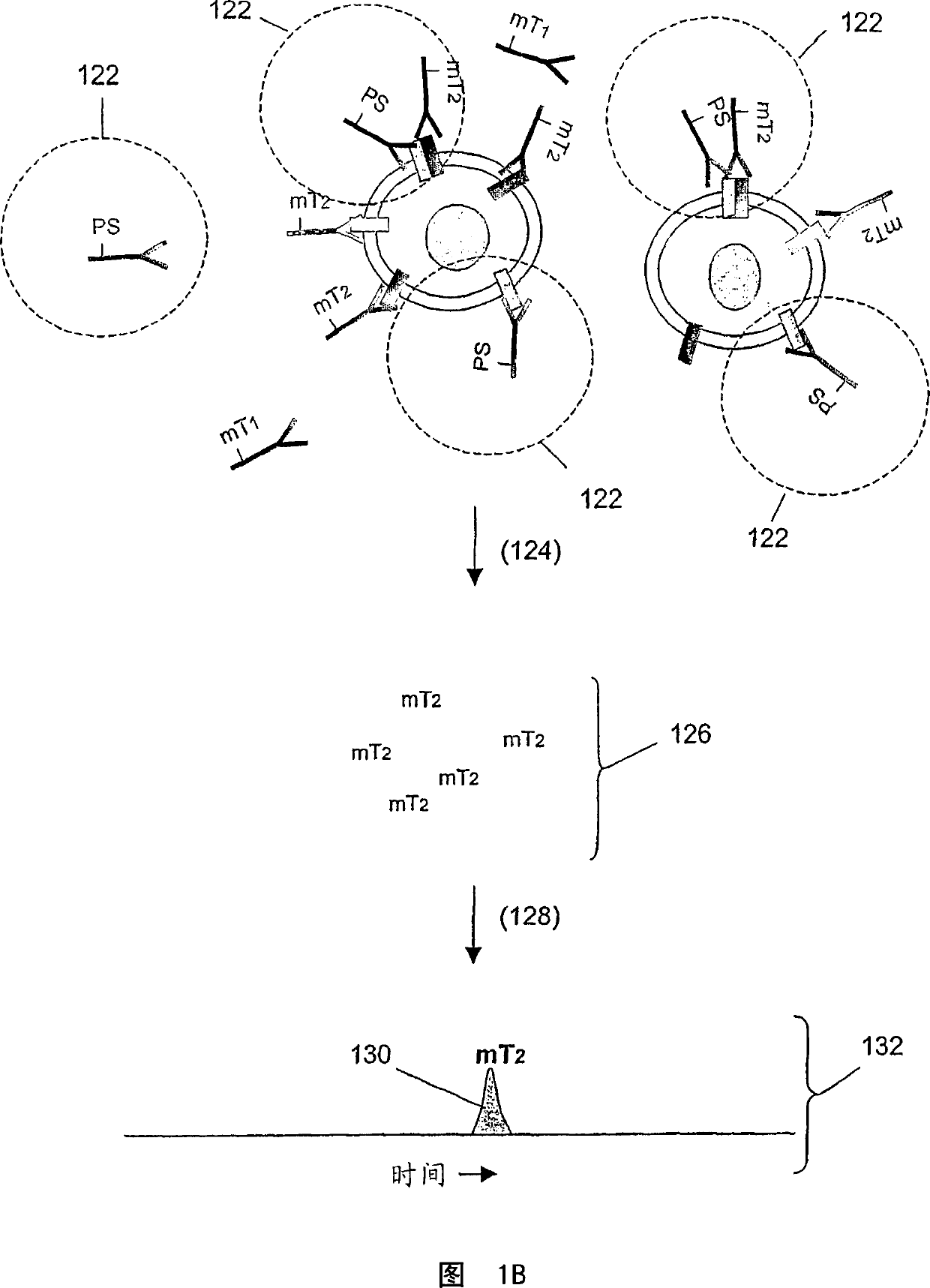
[0157] In this example, Her1-Her2 and Her2-Her3 heterodimers and phosphorylation status were determined in cell lysates from several cell lines treated with various concentrations of epidermal growth factor (EGF) and heregulin (HRG) were processed. Three binding compounds and cleavage probes were used for the measurements, as described below.
[0158] Sample Preparation:
[0159] 1. Serum-starved breast cancer cell lines were cultured overnight before use.
[0160] 2. Stimulate cell lines with EGF and / or HRG in culture medium for 10 minutes at 37°C. Doses of EGF / HRG such as 0, 0.032, 0.16, 0.8, 4, 20, 100nM for all cell lines (such as MCF-7, T47D, SKBR-3) except BT20, the maximum dose for BT20 should be increased to 500nM , because saturation is not achieved with 100 nM EGF.
[0161] 3. Aspirate medium, transfer to ice, and add lysis buffer to ly...
Embodiment 2
[0220] Her-2 heterodimerization on tissue lysates
[0221] and analysis of receptor phosphorylation
[0222] In this example, Her1-Her2 and Her2-Her3 heterodimers and phosphorylation status were determined in tissue lysates from human breast cancer samples.
[0223] Sample Preparation:
[0224] 1. Mechanically disrupt quick-frozen tissue in a frozen state by cutting.
[0225] 2. Transfer the tissue to a microcentrifuge tube and add 3x tissue volume of Lysis Buffer
[0226] (Appendix I), followed by agitation to disperse the tissue in the buffer.
[0227] 3. Incubate on ice for 30 minutes and stir occasionally.
[0228] 4. Centrifuge at 14000rpm for 20min at 4°C.
[0229] 5. Collect supernatant as lysate and use an aliquot to determine total protein concentration by BCA assay (Pierce)
[0230] 6. Aliquot the remaining material to store at -80°C until use.
[0231] Assay design:
[0232] 1. The total assay volume is 40ul.
[0233] 2. Test lys...
Embodiment 3
[0269] Her1 or Her2 homodimerization on cell lysates
[0270] and analysis of receptor phosphorylation
[0271] The procedure for sample preparation was essentially as described in Example 2. Cell lines were treated with EGF and TGFα to induce homodimerization of Her1. For homodimerization of Her2 without ligand, unstimulated SKBR-3 or MDA-MD453 cells overexpressing Her2 were compared to unstimulated MCF-7 cells expressing Her2 at low levels.
[0272] Assay design: Monoclonal antibodies specific to receptors are coupled to molecular tags or biotin (biotin is coupled to a photosensitizer through an avidin bridge), so that the cleavage probe and the binding compound compete Binds the same epitope as in this example. Another binding compound used includes a secondary antibody that recognizes overlapping epitopes on the receptor, thus generating a reference signal as a measure of homodimerization. The signal obtained from the secondary antibody al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com