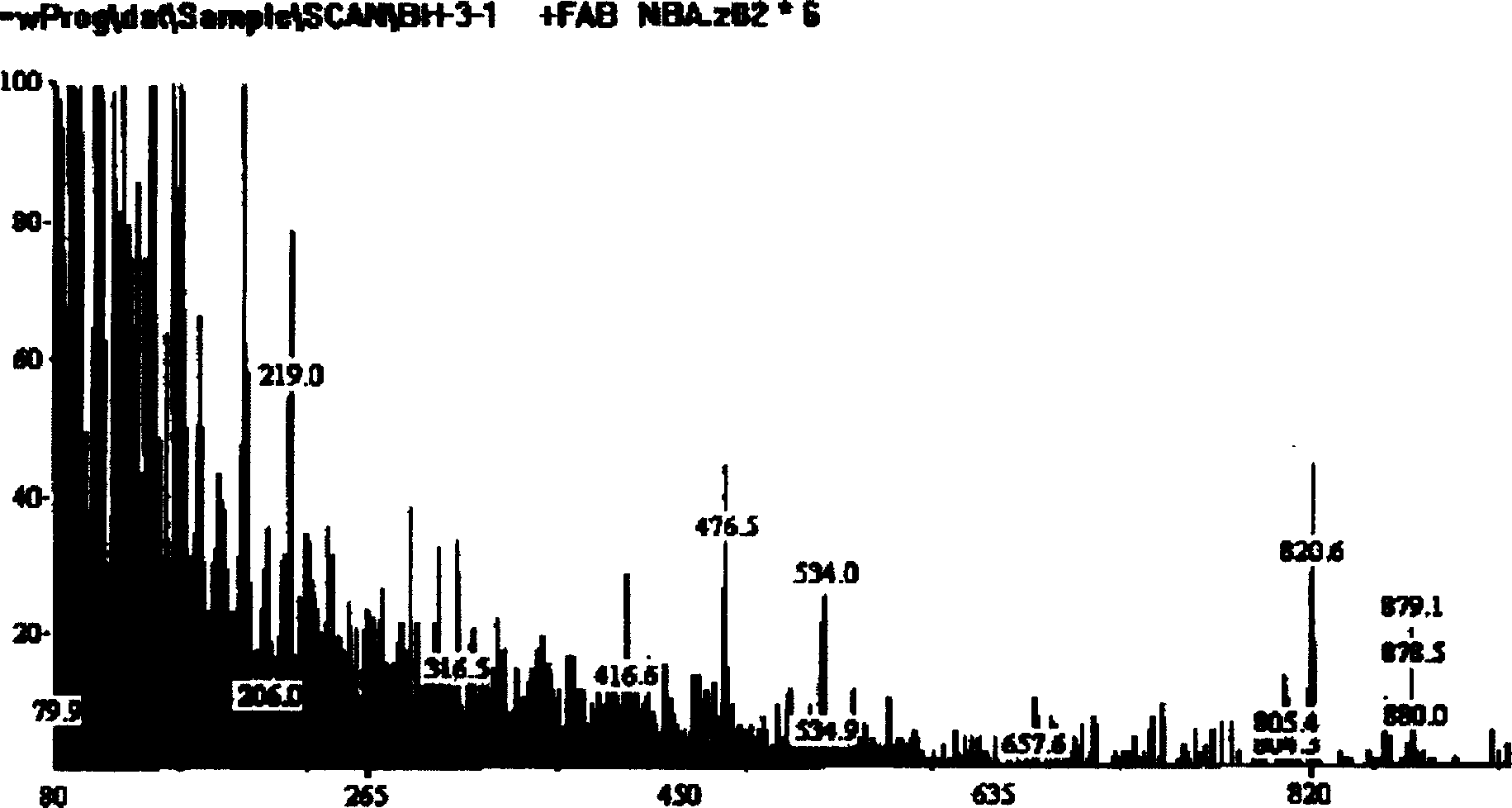
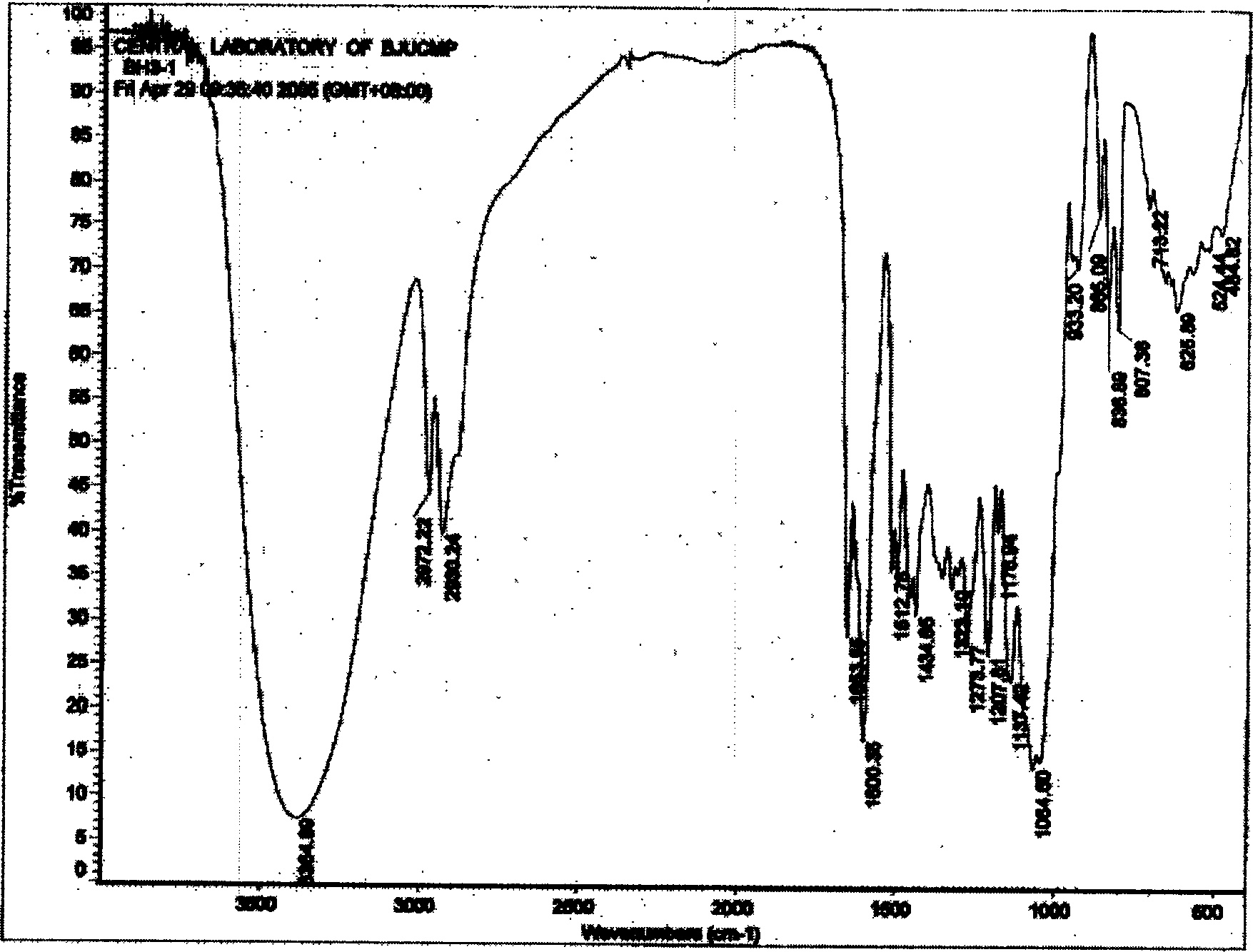
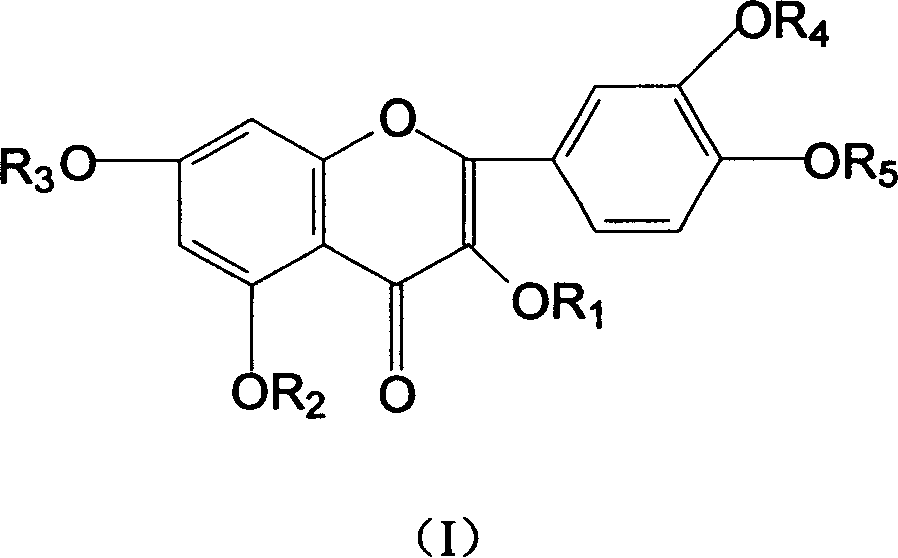
Hydroxy propyl rutin derivatives and preparation process thereof
A technology of hydroxypropyl rutin and derivatives, which is applied in the field of medicine and achieves the effects of broad market development prospects, low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] In a 500mL flask, add 5.5g NaOH and 250mL water, stir to dissolve. Then 46 g of rutin were added and stirred for 1.0 hour. 25 mL of propylene oxide was added dropwise with stirring, and the reaction was continued for 26 hours. Neutralize the reaction liquid with 2mol / L hydrochloric acid to neutral, desalt with ion exchange resin, concentrate under reduced pressure (20kPa), spray dry (not higher than 80°C), and obtain 38.6g of hydroxypropyl rutin.
Embodiment 2
[0040] In a 250mL flask, add 2.5gNaH, 200mL DMF (N,N-dimethylformamide), stir to dissolve. Then 12 g of partially methylated rutin were added and stirred until dissolved. 5 mL of 1-chloro-2-propanol was added dropwise with stirring, and the reaction was continued for another 30 hours. Neutralize the reaction liquid with 1mol / L sulfuric acid to neutrality, concentrate under reduced pressure, desalt and remove DMF with a chromatographic column, and dry in vacuum (20kPa) to obtain 9.2g of methyl-rutin containing hydroxypropyl components.
Embodiment 3
[0042] In a 250mL flask, add 1.5gKOH and 120mL water, stir to dissolve. Then 6 g of partially ethylated rutin was added and stirred until dissolved. 3 mL of propylene oxide was added dropwise with stirring, and the reaction was continued for 12 hours. Use 2mol / L acetic acid to neutralize the reaction solution to neutrality, extract the water phase through n-butanol extraction, heat the n-butanol phase under reduced pressure (5kPa) to recover n-butanol, and then vacuum-dry to obtain 4.2g hydroxypropyl Component ethyl-rutin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com