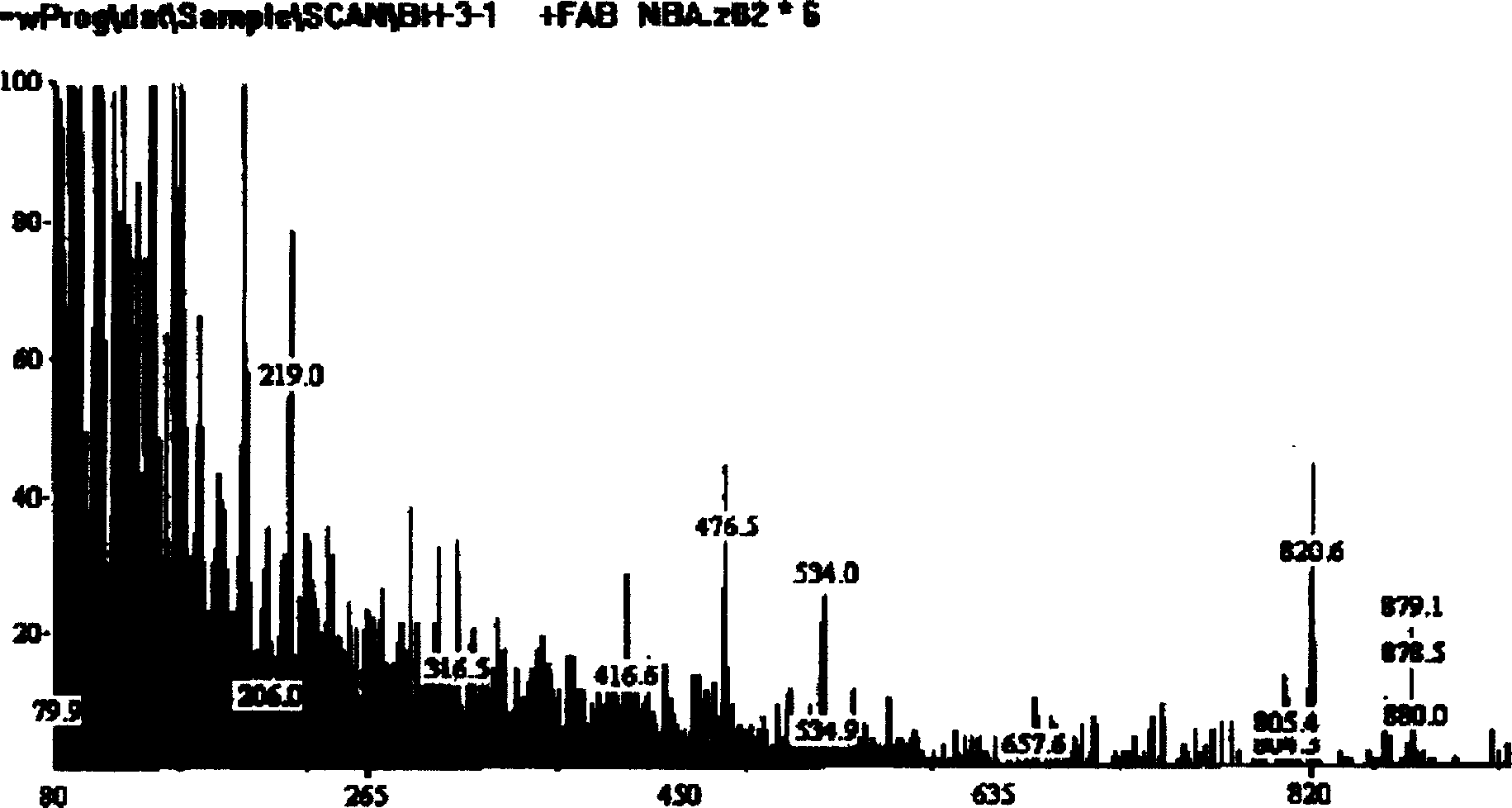
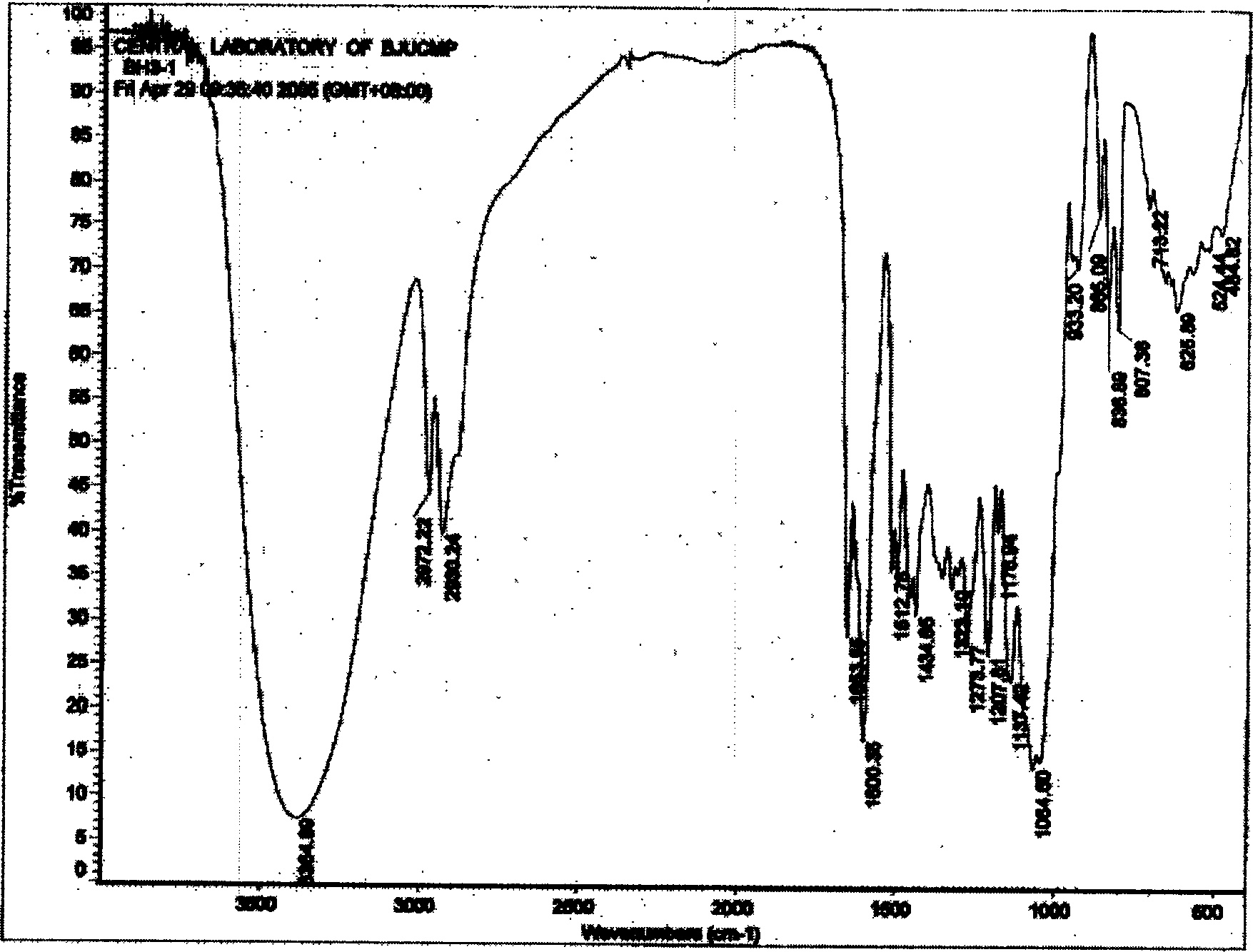
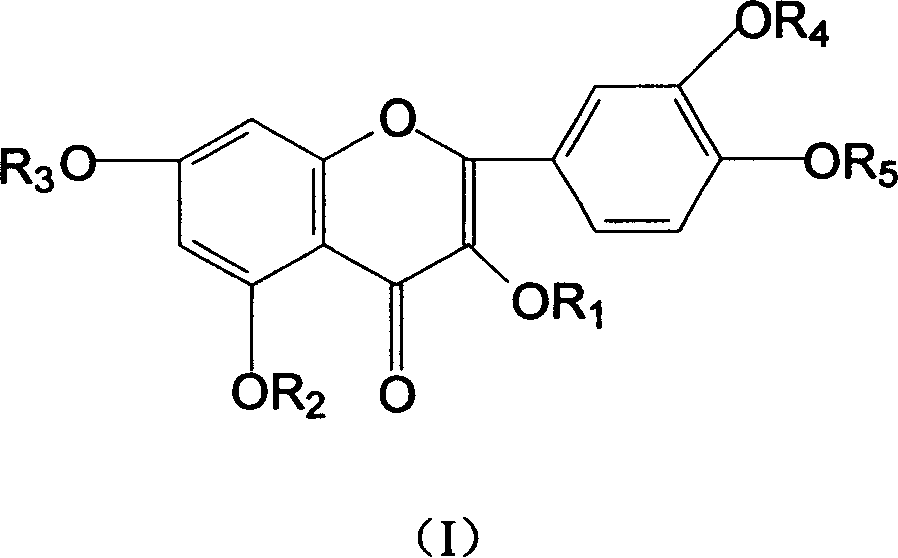
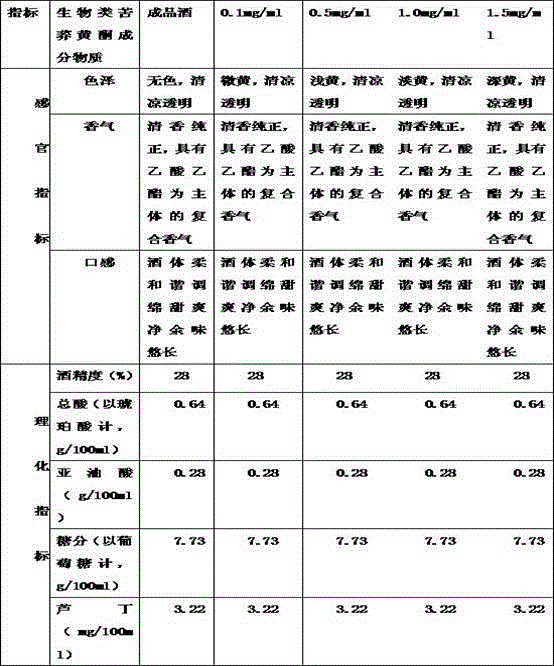
Patents
Literature
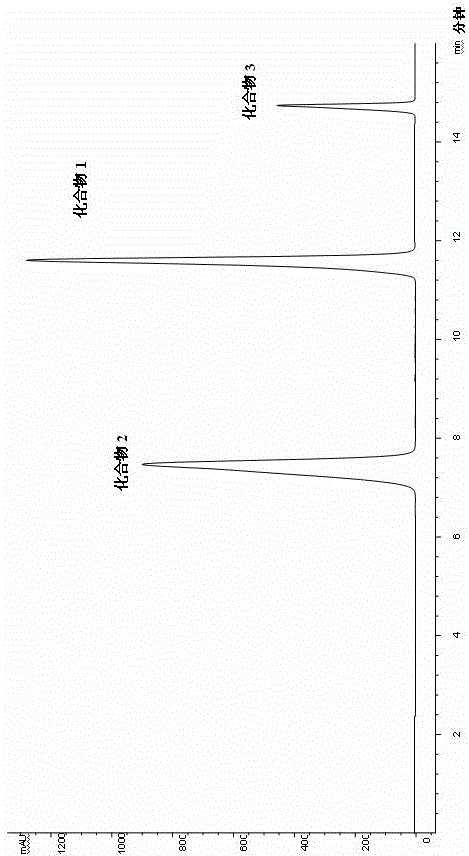
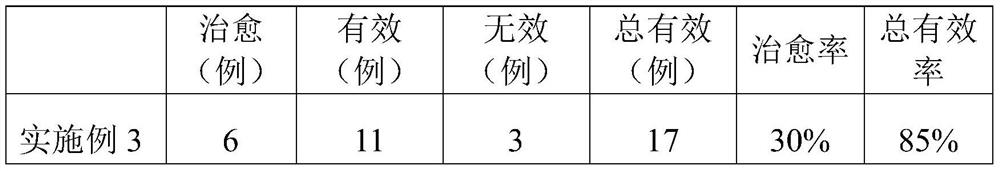
33 results about "Rutinose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
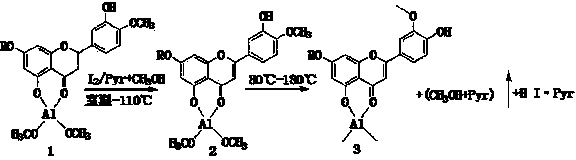
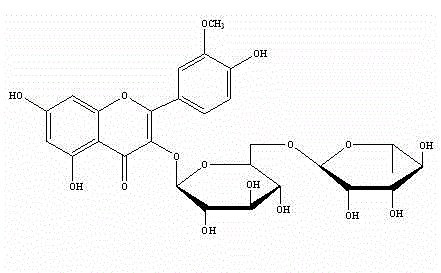
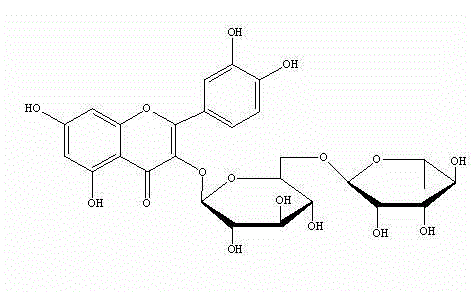
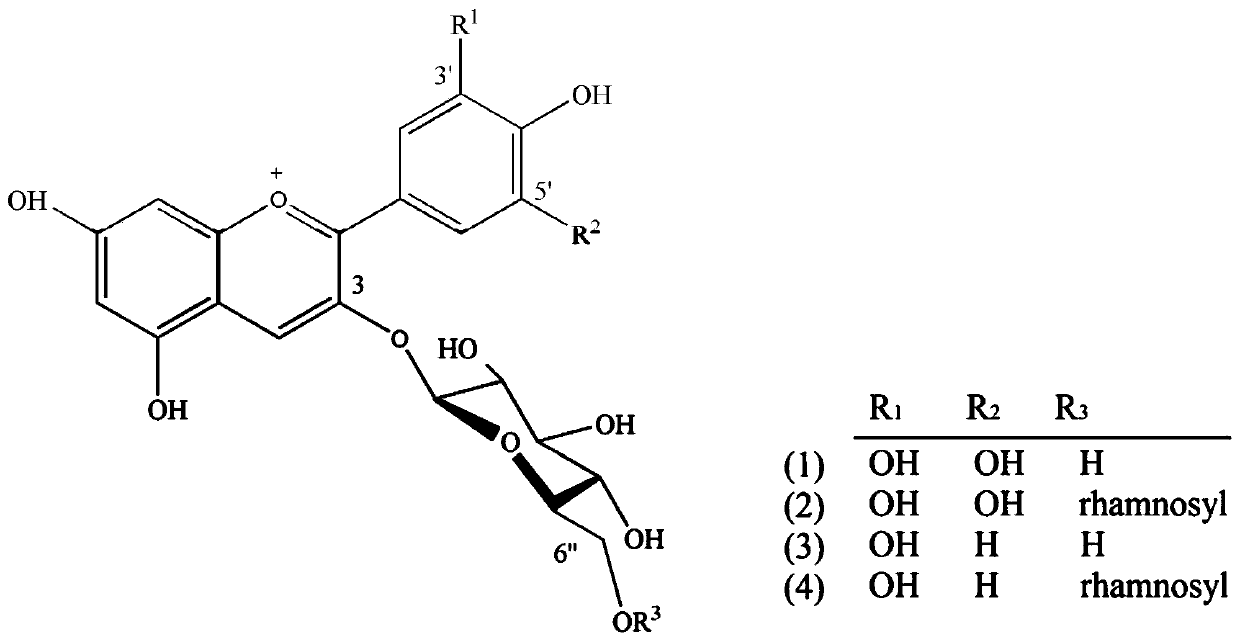
Rutinose is the disaccharide also known as 6-O-α-L-rhamnosyl-D-glucose (C₁₂H₂₂O₁₀) that is present in some flavonoid glycosides. It is prepared from rutin by hydrolysis with the enzyme rhamnodiastase.
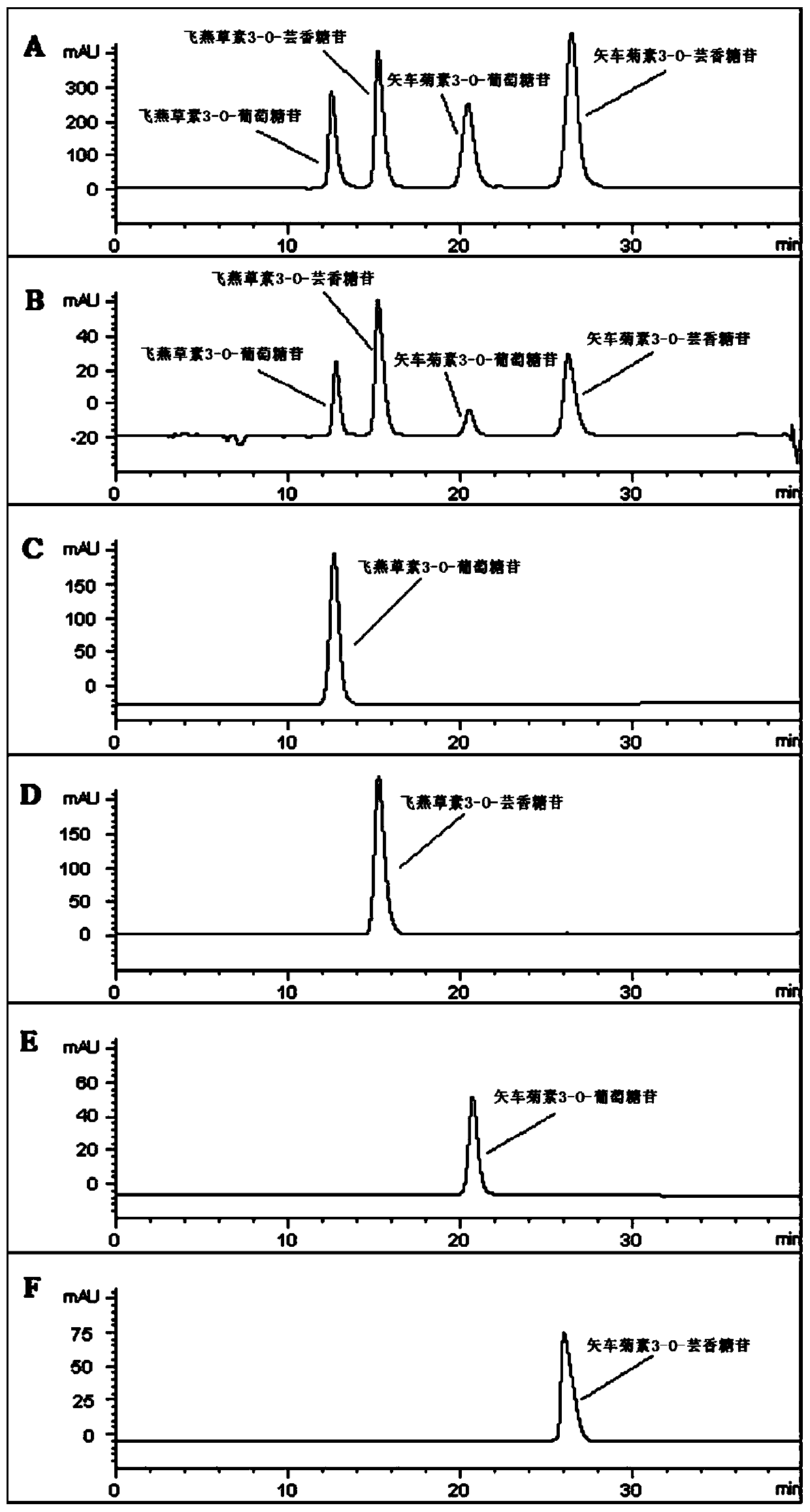
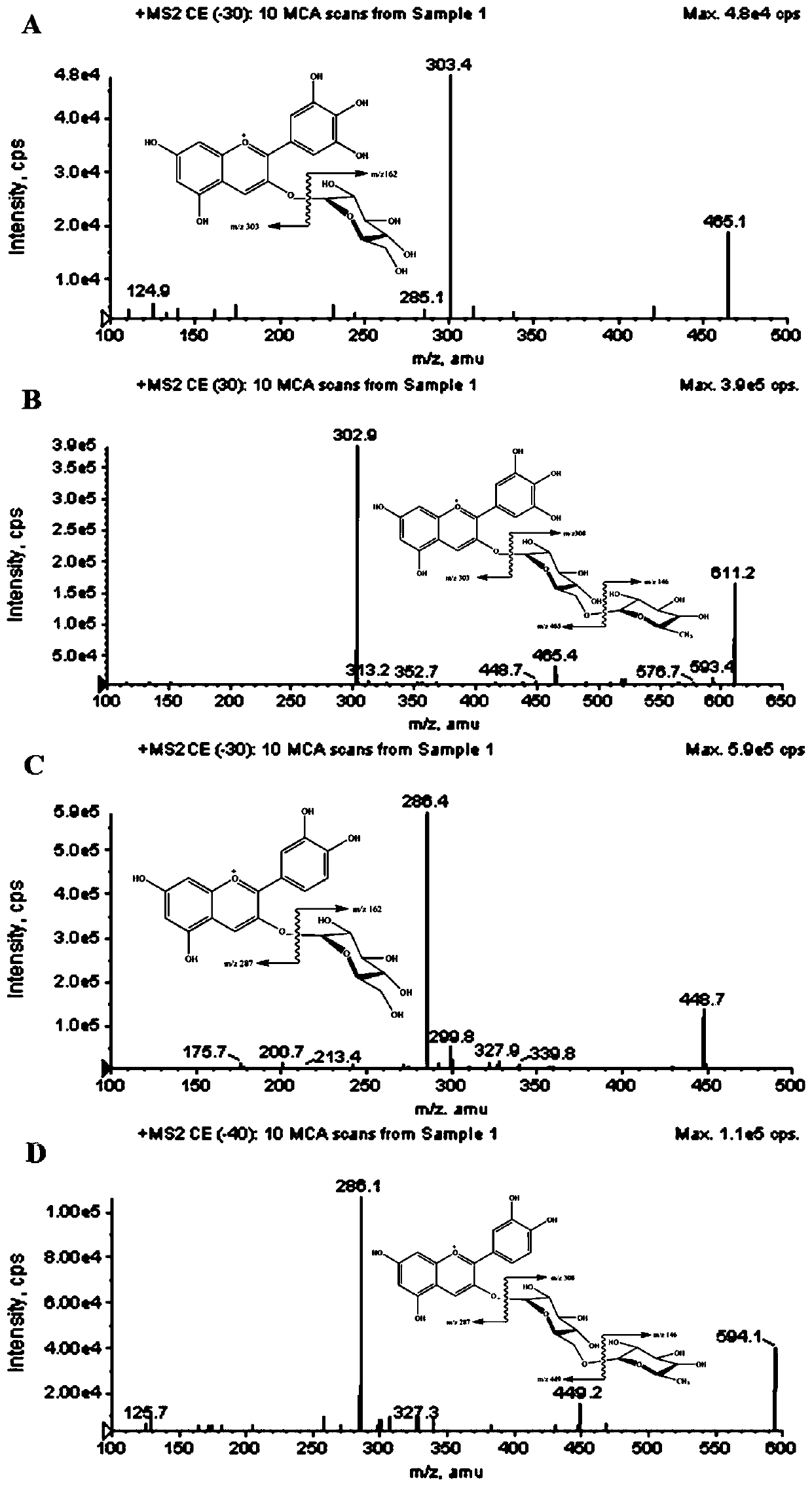
Method for extracting, separating and purifying four main anthocyanins from blackcurrant residue
ActiveCN107325138AHigh yieldIndustrial simplicitySugar derivativesSugar derivatives preparationChromatographic separationCavitation
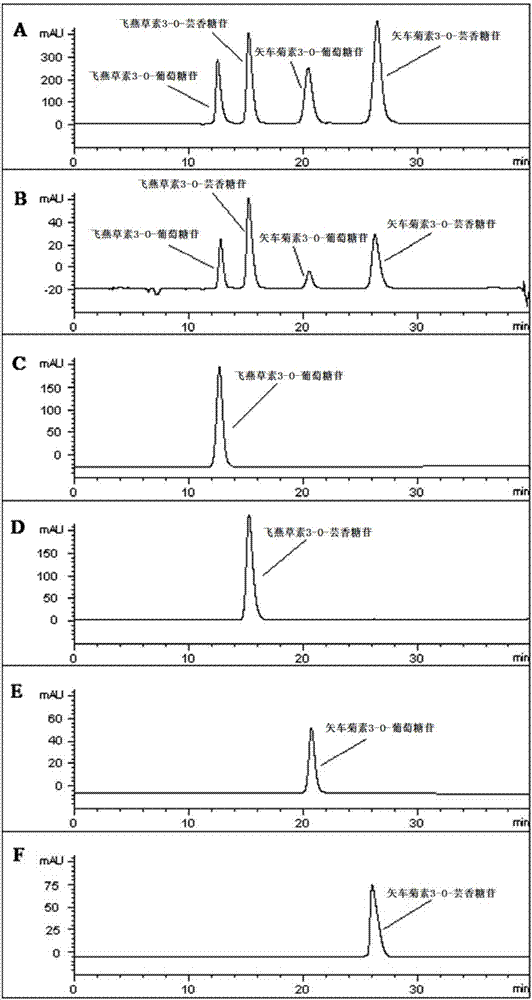
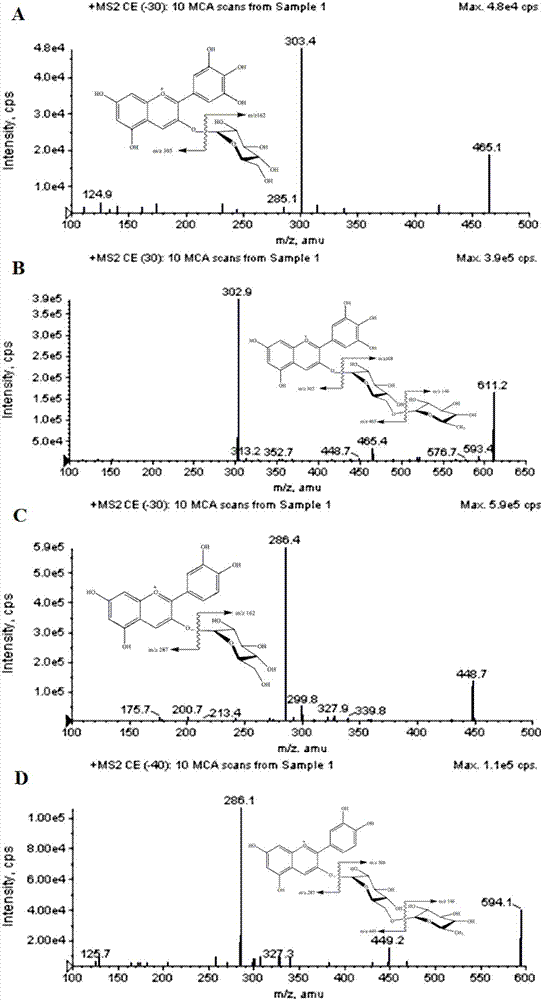
The invention relates to a method for extracting, separating and purifying four main anthocyanins from the blackcurrant residue. The object is to provide a simple, economic, green, eco-friendly, and high-yield method for extracting, separating and purifying four main high-purity anthocyanins from the blackcurrant residue. According to the method, the blackcurrant waste residue produced from industrial production is taken as a raw material, and homogenized pulverized coupling enzyme hydrolysis technology, negative-pressure cavitation reinforced extraction technology, macroporous adsorption resin concentration technology, medium-pressure quick-flash reverse chromatographic separation technology and low-temperature crystallization and recrystallization technology are adopted to obtain four anthocyanin monomer ingredients including delphinidin 3-O-glucoside, delphinidin 3-O-rutinoside, cyanidin 3-O-glucoside, and cyanidin 3-O-rutinoside and having the purity reach 95%. The method is abundant and available in raw materials, short in time consumption, less in solvent consumption, high in purity of obtained anthocyanins, low in production cost, and suitable for massive industrial production.
Owner:GREATER KHINGAN RANGE CHAOYUE WILD BERRY DEV
Method for preparing kaempferol-3-O-rutinoside from ginkgo leaf extract
ActiveCN103113436AHigh purityHigh degree of automationSugar derivativesSugar derivatives preparationKaempferol-3-O-rutinosideEvaporation
The invention provides a method for preparing kaempferol-3-O-rutinoside from a ginkgo leaf extract, which comprises the following steps: by using a two-dimensional liquid phase chromatography-mass spectroscopy combination technology and taking methanol-water or acetonitrile-water as a mobile phase and a reversed phase C18 chromatographic column as a one-dimensional preparative chromatographic column, performing component cutting on a ginkgo leaf extract, and collecting a target component which is a kaempferol-3-O-rutinoside crude product; and by taking a hydrophilic TECys as a two-dimensional preparative chromatographic column, performing component cutting on the kaempferol-3-O-rutinoside crude product, collecting, and concentrating through rotary evaporation to obtain the high-purity kaempferol-3-O-rutinoside, wherein the purity can be up to 80% or above. According to the invention, the preparation process is high in repetitiveness and favorable in operability; and meanwhile, ginkgo leaves are abundant in resources and easy to acquire. Thus, the invention meets the requirements for large-scale production and can be used for the preparation of raw materials for a Shuxuening injection.
Owner:北京华润高科天然药物有限公司
Process for preparing kaempferol derivatives
InactiveCN1629175AIncrease contentCultivation is simpleSugar derivativesSugar derivatives preparationGlucose polymersD-Glucose
The invention provides a novel process for preparing Kacmpferol derivative, i.e. Kacmpferol-3-0-rutinose glycoside and Kacmpferol-3-0-glucose glycoside monomer or their mixture by using the white flowers or light-yellow flowers of Carthamus tinctorius L. as raw material, extracting with water or water-containing ethanol, and isolating and purifying through microporous resin and silica gel column chromatography.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hydroxy butyl rutin derivatives and preparation process thereof
InactiveCN1837227AGood water solubilityImprove stabilitySugar derivativesSugar derivatives preparationIon exchangeRutin
The invention discloses a hudroxybutyl birutan derivant and preparing method, wherein the R1 is rutinose; R2,R3,R4,R5 is H or -CH, -C2H5, -CH2CH2OH, -CH2CH(OH)CH3, -CH2CH(OH)CH2CH3, -CH(CH3)CH(OH)CH3; at least one of R2, R3, R4, R5 is -CH2CH (OH) CH2CH3 or -CH (CH3) CH (OH) CH3. The preparing method comprises the following steps: mixing up with birutan or birutan derivant and epoxybutane to react; adopting one method of routine chromatogram, abstraction, hyperfiltration, ion exchange, electrodialysis, hyperfiltration to remove salt; drying to get yellow or light yellow powder hudroxybutyl birutan derivant.
Owner:SHANDONG NORMAL UNIV
Semi-synthesis method of luteolin and galuteolin as well as luteolin rutinoside
InactiveCN103833714AAvoid absorptionQuick joinSugar derivativesSugar derivatives preparationDehydrogenationHydrolysis
The invention relates to a semi-synthesis method of luteolin and galuteolin as well as luteolin rutinoside by using hesperidin, a semi-synthesis method of the galuteolin by using hesperetin glucoside, and a semi-synthesis method of the luteolin by using hesperetin, and belongs to the field of chemistry and medicines. The semi-synthesis method comprises the following steps: enabling the hesperidin, the hesperetin glucoside and the hesperetin to be subjected to complexation in pyridine alcohol fluid, dehydrogenizing by using iodine, directly distilling alcohol and pyridine, maintaining for a period of time in an airtight distilled state, and carrying out demethylation reaction, so that the hesperidin is generated into the luteolin rutinoside; generating the galuteolin by demethylation of diosmetin glucoside, and generating the luteolin by diosmetin; and hydrolyzing the luteolin rutinoside, so that the luteolin rutinoside is transformed into luteolin and galuteolin. The semi-synthesis method has the advantages that two steps of dehydrogenation and demethylation are combined into one step, and reaction conditions of dehydrogenation and demethylation are mild and easy to control; few reagents are used and green and environmentally friendly; and the demethylation yield is high, and the industrial production is easy. Compared with disclosed documents and patients, the semi-synthesis method has great advantages in the production of luteolin and glucosides of the luteolin.
Owner:迁西县板栗产业研究发展中心
Dried influenza vaccine preparation and method of producing the same
InactiveUS20160206728A1Improve distributionEasy to storeSsRNA viruses negative-senseOrganic active ingredientsAntigenMaltitol
The present invention provides a dried influenza vaccine preparation in which the activity of an influenza vaccine antigen can be stably maintained even when stored without strictly maintaining a low temperature, and which can be stably supplied. The present invention also provides a method of producing the dried influenza vaccine preparation. The present invention provides a dried influenza vaccine preparation containing an influenza vaccine antigen and a disaccharide, wherein the disaccharide is at least one selected from the group consisting of sucrose, maltose, palatinose, melibiose, isomalt, cellobiose, allolactose, isomaltose, sophorose, lactobionic acid, laminaribiose, xylobiose, turanose, gentiobiose, rutinose, kojibiose, nigerose, robinose, neohesperidose, sucralose, and maltitol.
Owner:NITTO DENKO CORP
Prediction method for content of raspberry kaempferol-3-O-rutinoside
PendingCN110674973AAchieve high quality and high priceForecastingTesting plants/treesEngineeringComputer science
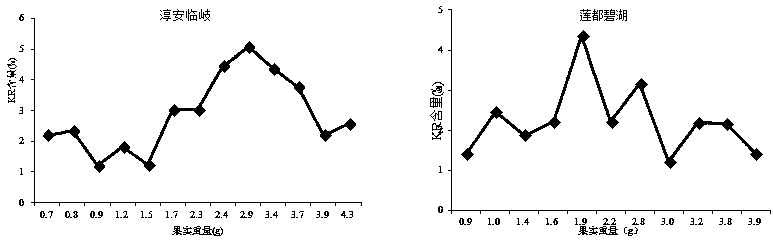
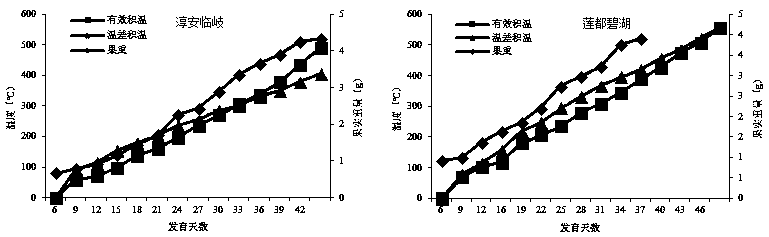
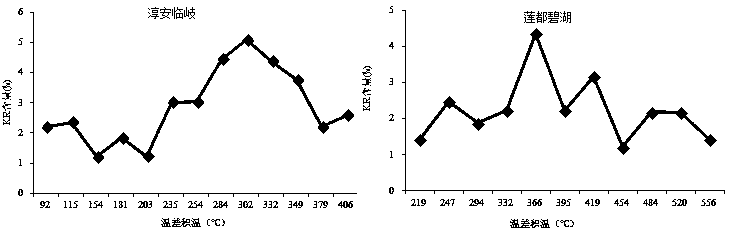
The invention discloses a prediction method for the content of raspberry kaempferol-3-O-rutinoside, and the method comprises the steps: building a prediction model through the temperature difference accumulated temperature and the dynamic changes of the index components of medicinal fruits, and achieving the dynamic prediction of the KR content based on the real-time accumulated temperature; and on the basis of the prediction model, secondary screening is carried out by referring to the fruit weight, so that the screening accuracy is improved. A raspberry medicinal fruit high-quality harvesting decision-making system is established through the prediction method, harvesting period prediction of a production place is given, it is guaranteed that the content of kaempferol-3-O-rutinoside reaches the standard, high quality and high price are achieved, benefits of enterprises and farmers are balanced, and technical support is provided for raspberry industry improvement.
Owner:ZHEJIANG ACADEMY OF AGRICULTURE SCIENCES
Method for enriching beta-ethyoxyl rutinose
InactiveCN103755751ASugar derivativesSugar derivatives preparationMacroporous resinColumn chromatography
The invention discloses a method for enriching beta-ethyoxyl rutinose. The method specifically comprises the following steps that an enzymatic converted tartary buckwheat raw material with high beta-ethyoxyl rutinose content is extracted in ethanol, is depressurized to concentrate, is subjected to column chromatography by using a macroporous resin, and is further enriched by using an MCI column. The content of beta-ethyoxyl rutinose in a final extract is greater than 20%.
Owner:山西春阳生物科技股份有限公司
Extraction separation method for isorhamnetin-3-O-beta-D-rutinoside in caragana sinica flower bud
InactiveCN104130299AThe method is simple and feasibleEasy to produceSugar derivativesSugar derivatives preparationBiotechnologyPolyamide
The invention discloses an extraction separation method for isorhamnetin-3-O-beta-D-rutinoside in caragana sinica flower bud. The method comprises: performing crushing, refluxing extraction and concentration on dry caragana sinica flower buds, so as to obtain a crude extractive; firstly performing extraction segmentation on the crude extractive, selecting solvent systems petroleum ether, ethyl acetate and n-butanol, performing separation elution on the n-butanol-containing extraction part through polyamide phenol resin, concentrating the eluate until no alcohol smell exists, cooling, standing, and filtering to obtain isorhamnetin-3-O-beta-D-rutinoside. The content of isorhamnetin-3-O-beta-D-rutinoside prepared by employing the method is larger than 80%. The method is high in extraction rate, low in equipment requirement and easy for expanded production, and is suitable of industrialized production.
Owner:KUNMING UNIV OF SCI & TECH
Ginkgo leaf flavone extract as well as preparation method and characteristic spectrum construction method thereof
ActiveCN113995776AHigh purityThe effect component is clearComponent separationAntinoxious agentsBiotechnologyGinkgo biloba
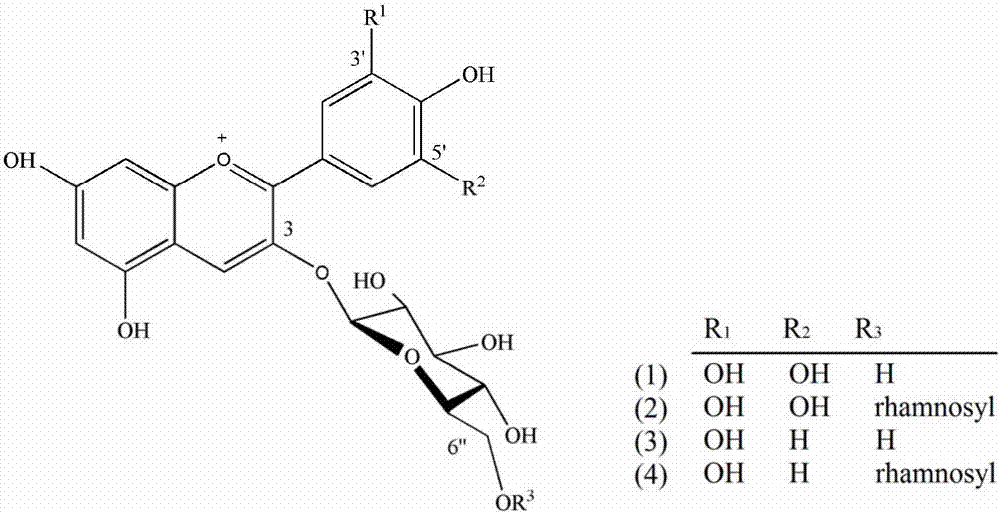
The invention provides a ginkgo leaf flavone extract as well as a preparation method and a characteristic spectrum construction method thereof, the ginkgo leaf flavone extract comprises the following medicinal components: 3%-6% (w / w) of myricetin-3-O-rutinose, 3%-6% (w / w) of myricetin-3-O-glucose, 8%-11% (w / w) of rutin, 5%-7% (w / w) of tageted-3-O-rutinose, 5%-7% (w / w) of quercetin-3-O-rutinose, 6%-9% (w / w) of kaempferol-3-O-rutinose, 6%-10% (w / w) of isorhamnetin-3-O-rutinose, 13%-15% (w / w) of quercitrin-2''-(6''-p-cinnamyl glucosyl) and 11%-15%(w / w) of kaempferol-3-(6''-p-cinnamyl glucosyl-rhamnoside). The flavone extract is obtained by twice separation of a ginkgo leaf extract through a polyamide column, and can be used as an antioxidant in food, medicines and health care products. According to the method, the problem that the effect components of the ginkgo leaf flavone extract are unknown is solved, the quality control difficulty is reduced, and the established ultra-high performance liquid characteristic chromatogram has specificity for quality detection.
Owner:ZHEJIANG CONBA PHARMA
Hydroxy propyl rutin derivatives and preparation process thereof
InactiveCN1837228AGood water solubilityImprove stabilitySugar derivativesSugar derivatives preparationIon exchangeRutin
The invention discloses a hudroxybutyl birutan derivant and preparing method, wherein the R1 is rutinose; R2,R3,R4,R5 is H or -CH3, -C2H5, -CH2CH2OH or -CH2CH(OH)CH3; at least one of R2, R3, R4, R5 is -CH2CH(OH)CH. The preparing method comprises the following steps: mixing up with birutan or birutan derivant and epoxypropane or 1-chlorine-2-optal to react; adopting one method of routine chromatogram, extraction, hyperfiltration, ion exchange, electrodialysis and hyperfiltration to remove salt; drying to get yellow or light yellow powder hydroxypropyl birutan derivant.
Owner:SHANDONG NORMAL UNIV
Antioxidative tartary buckwheat and oat liquor and making method thereof
InactiveCN105039086AAddressed an issue where damage drinking was not working wellAntioxidantAlcoholic beverage preparationBiotechnologyFagopyrum tataricum
The invention relates to antioxidative tartary buckwheat and oat liquor which is made from, by weight, tartary buckwheat, oat, sorghum, yeast for making hard liquor, rice chaff, pure water and biological tartary buckwheat flavone class substance. Each part of the biological tartary buckwheat flavone substance is composed of rutin, quercetin, kaempferol, kaempferol-3-rutinoside, quercetin-3-rutinoside, heteroside and isoquercetin. The making method includes the steps that firstly, tartary buckwheat and oat unblended liquor is brewed; then, biological tartary buckwheat and flavone substance is extracted by decocting tartary buckwheat grains and tartary buckwheat roots to be added back to the unblended liquor, and then fermentation and brewing are conducted; thirdly, 60-degree tartary buckwheat and oat unblended liquor is brewed; the unblended liquor obtained in the above steps is blended, filtered and then stored in a stainless steel tank for use; fourthly, laboratory test and measuring, blending or lowering the alcoholic strength and finished product packaging are conducted. The antioxidative tartary buckwheat and oat liquor has obvious functions of lowering blood glucose, lowering lipid, removing free radical and enhancing immunity and the like.
Owner:山西壶泉苦荞酒业有限公司
Dried influenza vaccine preparation and method for producing dried influenza vaccine preparation
InactiveCN105530955AMaintain activity stablyStable supplySsRNA viruses negative-sensePowder deliveryAntigenMaltitol
The purpose of the present invention is to provide the following: a dried influenza vaccine preparation with which stable retention of the activity of influenza vaccine antigen is possible, even when the preparation is stored without strict low-temperature management, and with which a stable supply is possible; and a method for producing the dried influenza vaccine preparation. The present invention provides a dried influenza vaccine preparation comprising influenza vaccine antigen and a disaccharide, wherein the disaccharide is one or more selected from the group consisting of sucrose, maltose, palatinose, melibiose, isomalt, cellobiose, allolactose, isomaltose, sophorose, lactobionic acid, laminaribiose, xylobiose, turanose, gentiobiose, rutinose, kojibiose, nigerose, robinose, neohesperidose, sucralose, and maltitol.
Owner:NITTO DENKO CORP
Detection method of gingko flavonol glycosides
ActiveCN112697899AAccurately reflectEffective reflectionComponent separationGlycosideDrugs preparations
The invention discloses a detection method of gingko flavonol glycosides. The detection method comprises the following steps of: (1) preparing reference substances and a test solution, the reference substances being rutin, hyperoside, isoquercitrin, quercetin-3-O-2''-glucose rhamnoside, kaempferol-3-O-beta-D-rutinoside, narcissoside, syringetin-3-O-beta-D-rutinoside, cosmosiin, quercetin-3-O-p-coumaroyl rhamnose glucoside and kaempferol-3-O-p-coumaroyl rhamnose glucoside reference substances; (2) performing detection; (3) establishing a standard fingerprint spectrum; and (4) calculating the content of common peaks in the fingerprint spectrum established in the step (3). According to the detection method, a specific standard fingerprint spectrum of the ginkgo flavonol glycosides is established, the content of main flavonol glycosides in gingko and related extracts and pharmaceutical preparations thereof can be accurately detected, and the accuracy, precision, reproducibility and stability are good.
Owner:CHINA PHARM UNIV
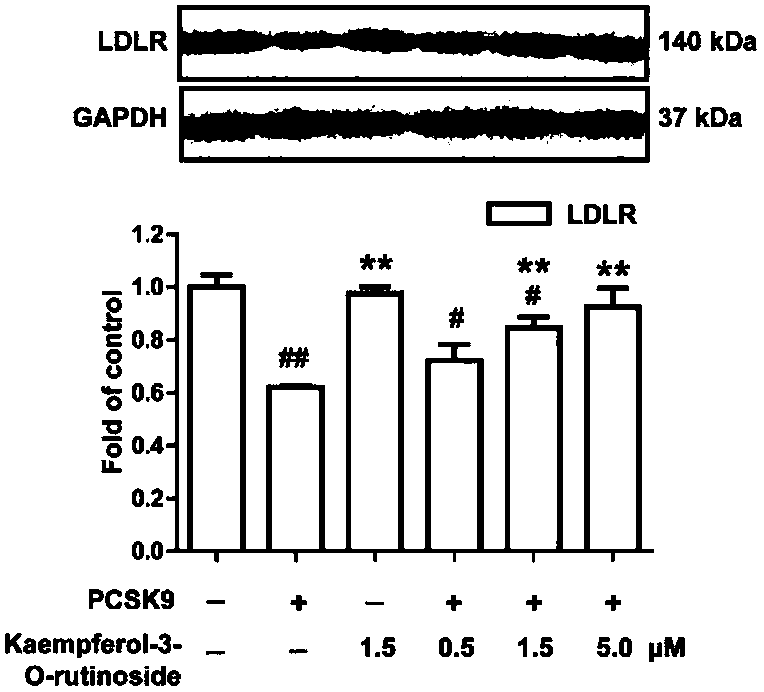
Application of kaempferol-3-O-rutinoside to preparation of medicine for treating PCSK9 (Proprotein convertase subtilisin/kexin type 9)-mediated disease
InactiveCN108567788AInhibit bindingImprove lipid metabolism disorderOrganic active ingredientsMetabolism disorderKaempferol-3-O-rutinosideKexin
The invention relates to the lipid-lowering field of kaempferol-3-O-rutinoside, and relates to application of kaempferol-3-O-rutinoside to preparation of PCSK9 (Proprotein convertase subtilisin / kexintype 9) target inhibition medicine. Kaempferol-3-O-rutinoside is firstly found to have specific activity for inhibiting the binding of PCSK9 to LDLR (Low-Density Lipoprotein Receptor), can be subjected to affinity binding with an active pocket area of a PCSK9 crystal structure, and form stable hydrogen bonds with a plurality of amino acid residues, thereby being a novel small molecule inhibitor for PCSK9. The kaempferol-3-O-rutinoside can reduce the levels of TC (Total Cholesterol), TG (Triglyceride) and LDL-C (Low-Density Lipoprotein Cholesterol) in plasma of hyperlipidemia model mice, and improve the level of HDL-C (High-Density Lipoprotein cholesterol). The kaempferol-3-O-rutinoside has the effect of alleviating lipid metabolism disorder of hyperlipidemia and can be used for preventingor treating cardiovascular diseases (including the hyperlipidemia).
Owner:CHINA PHARM UNIV
Method for preparing quercitrin and kaempferol-3-O-rutinoside from Chinese redbud leaf extract
ActiveCN113717140AHigh extraction ratePromote development and utilizationSugar derivativesSugar derivatives preparationSephadexGlycoside
The invention discloses a method for preparing quercitrin and kaempferol-3-O-rutinoside from a Chinese redbud leaf extract, and belongs to the technical field of medicines. The method comprises the following steps: taking dried Chinese redbud leaves as raw materials, smashing the leaves, then extracting the leaves with 75% ethyl alcohol at the temperature of 50 DEG C, subjecting the extract to vacuum concentration, recovering the solvent, and obtaining a total extract; adding water to dissolve the total extract, and sequentially extracting with petroleum ether, dichloromethane and ethyl acetate to obtain different organic layers; carrying out vacuum concentration on the ethyl acetate layer, adding water into the obtained extract for dissolving, making the extract flow through AB-8 macroporous adsorption resin, and carrying out gradient elution by using ethanol with different concentrations to obtain corresponding elution parts; and carrying out vacuum concentration on the 60% ethanol elution part, dissolving with methanol, passing through a Sephadex LH-20 gel chromatographic column, and eluting with methanol to obtain a pure product of quercitrin and kaempferol-3-O-rutinoside. The extraction process is simple, a pure product of quercitrin and kaempferol-3-O-rutinoside is efficiently obtained, the used separation materials and solvents can be recycled, and the method is green, environmentally friendly, large in extraction amount and suitable for industrial production.
Owner:XUCHANG UNIV +2
Extraction and Separation of Rhamnetin-3-o-β-d-Rutinose from Gorse Flower Buds
InactiveCN104130299BThe method is simple and feasibleEasy to scale up productionSugar derivativesSugar derivatives preparationCytisus scopariusBud
The invention discloses isorhamnetin-3-O- β - The extraction and separation method of D-rutin, the dried gorse flower buds are crushed, refluxed, extracted, and concentrated to obtain a crude sample of the extract; the crude sample of the extract is first extracted and sectioned, and the solvent system is petroleum ether and ethyl acetate And n-butanol, the extracted part containing n-butanol is further separated and eluted by polyamide phenol resin column, the eluate is concentrated until it has no alcohol smell, the pH is adjusted to 2~3, cooled and stood, and filtered to obtain isorhamnetin-3 -O- β -D-rutinose; the isorhamnetin-3-O- that the inventive method makes β -D-rutinose content > 80%, the method has a high extraction rate, low equipment requirements, easy to expand production, and is suitable for industrial production.
Owner:KUNMING UNIV OF SCI & TECH
Method for extracting myricetin-3-O-rutinose from taxus mairei leaves
ActiveCN103030673ASimple extraction methodEasy to implementSugar derivativesSugar derivatives preparationAlcoholPolyamide
The invention discloses a method for extracting myricetin-3-O-rutinose from taxus mairei leaves. The method comprises the steps of using a supercritical extraction kettle to extract, using methyl alcohol to absorb, using AB-8 resin to conduct chromatography, using 100-mesh polyamide to conduct the chromatography, collecting an eluent at 6-9min, concentrating and drying, and obtaining a white crystallized compound of myricetin-3-O-rutinose. The extracting method is simple and easy to implement, and the purity of myricetin-3-O-rutinose in the extracted white crystallized compound reaches 99.76%.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
A kind of anthocyanin and use thereof
ActiveCN106924278BIncrease consumptionLower fasting blood sugarOrganic active ingredientsSugar derivativesDiseaseFasting glucose
The invention discloses rubus hirsutus anthocyanin and application thereof. The rubus hirsutus anthocyanin mainly comprises cyanidin-3-O-glucoside, pelargonine 3-O-glucoside and pelargonine 3-O-rutinoside, wherein the pelargonine 3-O-glucoside is main anthocyanin. An animal experiment proves that the rubus hirsutus anthocyanin can significantly reduce fasting blood glucose of db / db diabetic mice and shows excellent hypoglycemic activity in vivo. An in-vitro experiment proves that the rubus hirsutus anthocyanin can play a hypoglycemic role by inhibiting the alpha-glucosidase activity and promoting the glucose consumption of cells. Therefore, the rubus hirsutus anthocyanin can be taken as hypoglycemic functional food or a hypoglycemic drug for the prevention and the treatment of glycometabolism disorder-related diseases. The invention provides a new idea for comprehensive development and utilization of rubus hirsutus resources in China.
Owner:ZHEJIANG UNIV +1
A preparation method of freeze-dried liposome containing hair growth composition
ActiveCN112120953BGood hair growthUniform particle sizeCosmetic preparationsHair cosmeticsLipid filmCholesterol
The present invention relates to a preparation method of freeze-dried liposomes containing a hair growth composition, which is characterized in that the following steps are adopted: A. Weighing Suihua Shan double Flavonoids, quercetin-3-o-rutinoside, tetramethylpyrazine, and ricinoleic acid form a hair growth composition, and weigh hyaluronic acid oligosaccharides and sclerotin according to a mass ratio of 1:0.2-0.5 Composing a lyoprotectant; B, dissolving the hair growth composition, cholesterol and hydrogenated lecithin in ethanol, and removing the alcohol by rotary evaporation under reduced pressure after ultrasonic treatment, to obtain a lipid film containing the hair growth composition; C, adding the above lipid Add isotonic phosphate buffer solution to the membrane, sonicate and filter to obtain a liposome suspension; D, add a lyoprotectant to the above liposome suspension, freeze at -50~-10°C, vacuum for 36-72 h, the freeze-dried liposome containing the hair growth composition is obtained. The obtained freeze-dried liposome containing the hair growth composition has the advantages of uniform particle size, good stability, convenient use and good hair growth effect.
Owner:PROYA COSMETICS
Method for extracting isorhamnetin 3-o-rutinoside from Suaeda salsa
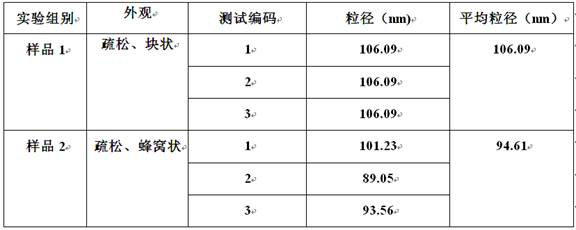
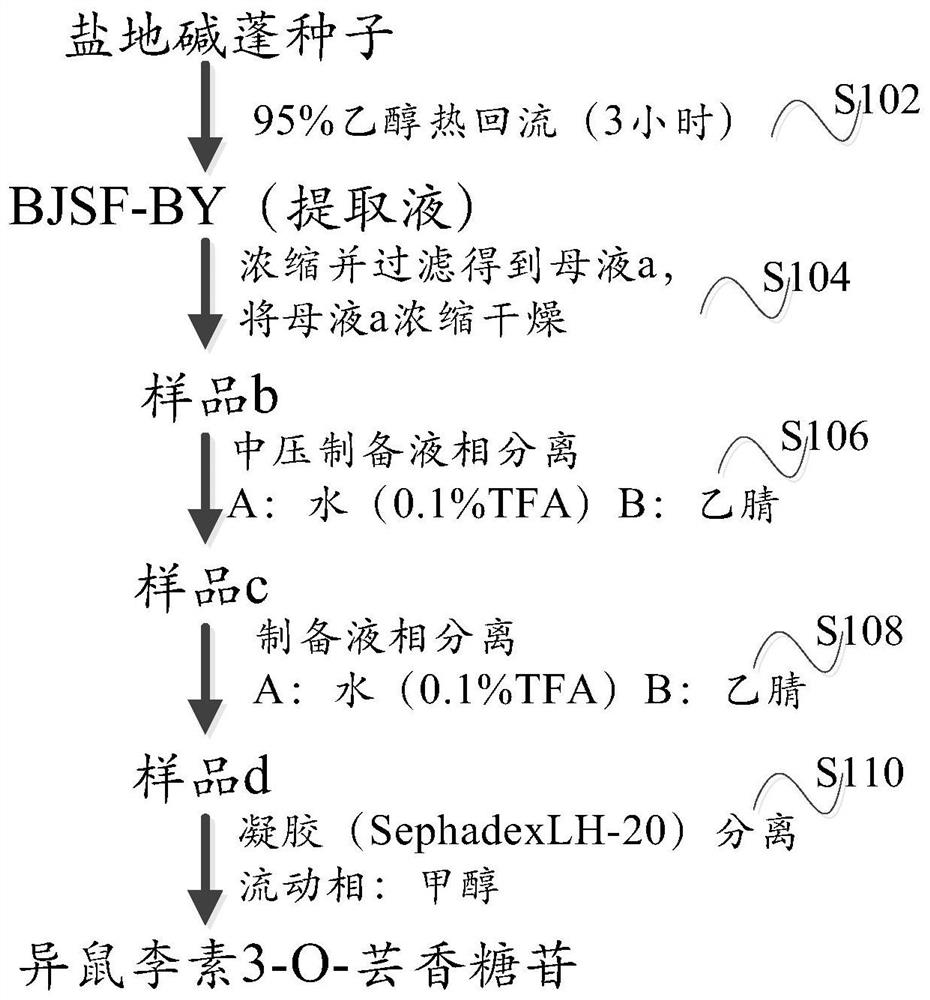
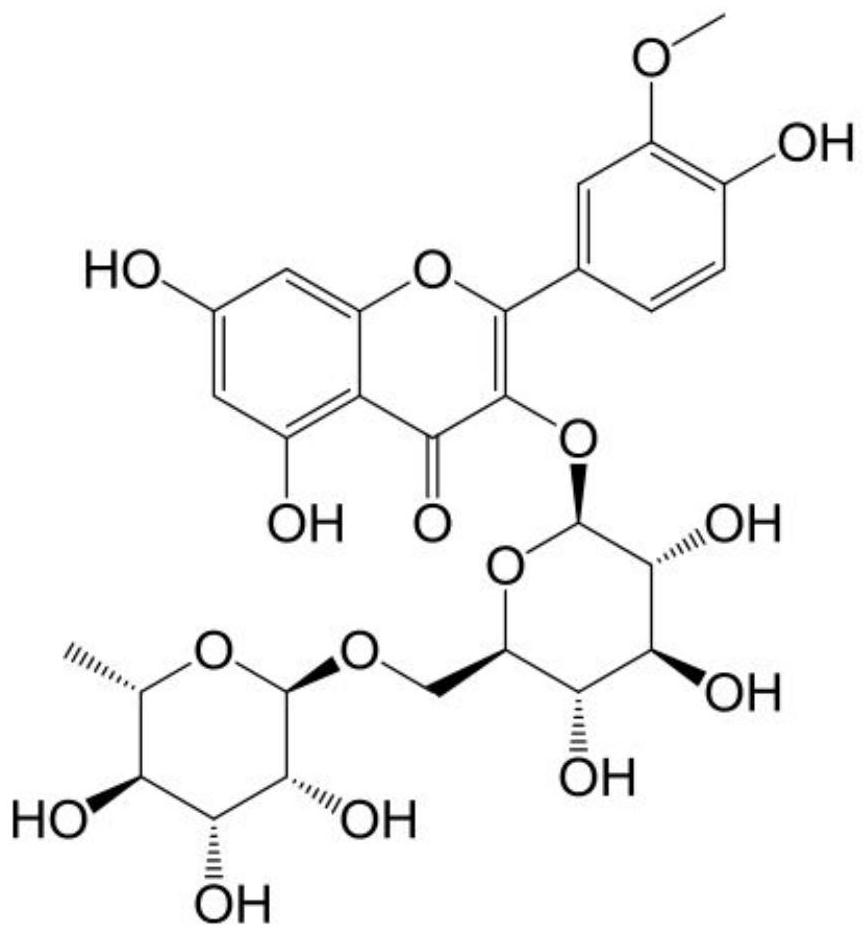
ActiveCN111718385BFully develop resourcesSafe and easy extractionSugar derivativesSugar derivatives preparationBiotechnologyFluid phase
The invention provides a method for extracting isorhamnetin 3-O-rutinoside from Suaeda salsa, comprising the following steps: (1) soaking the collected Suaeda salsa seeds for pretreatment; (2) using 95 Extracting Suaeda salsa seed extract with % ethanol under heat reflux, concentrating and filtering to obtain mother liquor a; (3) concentrating and drying said mother liquor a to obtain sample b; (4) dissolving said sample b with a mixture of ethanol and tetrahydrofuran , after filtration, use medium pressure preparative liquid phase separation to obtain fraction c; (5) dissolve the fraction c with a mixture of water and acetonitrile, and use preparative liquid phase separation after filtration to obtain fraction d; (6) dissolve the fraction c with methanol Fraction d, using methanol as the mobile phase, was separated by gel to obtain solid powder isorhamnetin 3‑O‑rutinoside. Through the technical scheme of the invention, the extraction of isorhamnetin 3-O-rutinoside from Suaeda salsa is realized, the extraction process is safe and convenient, and the purity can reach 99.5%.
Owner:BEIJING NORMAL UNIVERSITY
A method for preparing kaempferol-3-o-rutinoside from ginkgo biloba extract
ActiveCN103113436BHigh purityHigh degree of automationSugar derivativesSugar derivatives preparationBiotechnologyGinkgo Biloba Leaf Extract
The invention provides a method for preparing kaempferol-3-O-rutinoside from a ginkgo leaf extract, which comprises the following steps: by using a two-dimensional liquid phase chromatography-mass spectroscopy combination technology and taking methanol-water or acetonitrile-water as a mobile phase and a reversed phase C18 chromatographic column as a one-dimensional preparative chromatographic column, performing component cutting on a ginkgo leaf extract, and collecting a target component which is a kaempferol-3-O-rutinoside crude product; and by taking a hydrophilic TECys as a two-dimensional preparative chromatographic column, performing component cutting on the kaempferol-3-O-rutinoside crude product, collecting, and concentrating through rotary evaporation to obtain the high-purity kaempferol-3-O-rutinoside, wherein the purity can be up to 80% or above. According to the invention, the preparation process is high in repetitiveness and favorable in operability; and meanwhile, ginkgo leaves are abundant in resources and easy to acquire. Thus, the invention meets the requirements for large-scale production and can be used for the preparation of raw materials for a Shuxuening injection.
Owner:北京华润高科天然药物有限公司
Neutralizing agent for clostridium bacterial neurotoxins and preparation method thereof
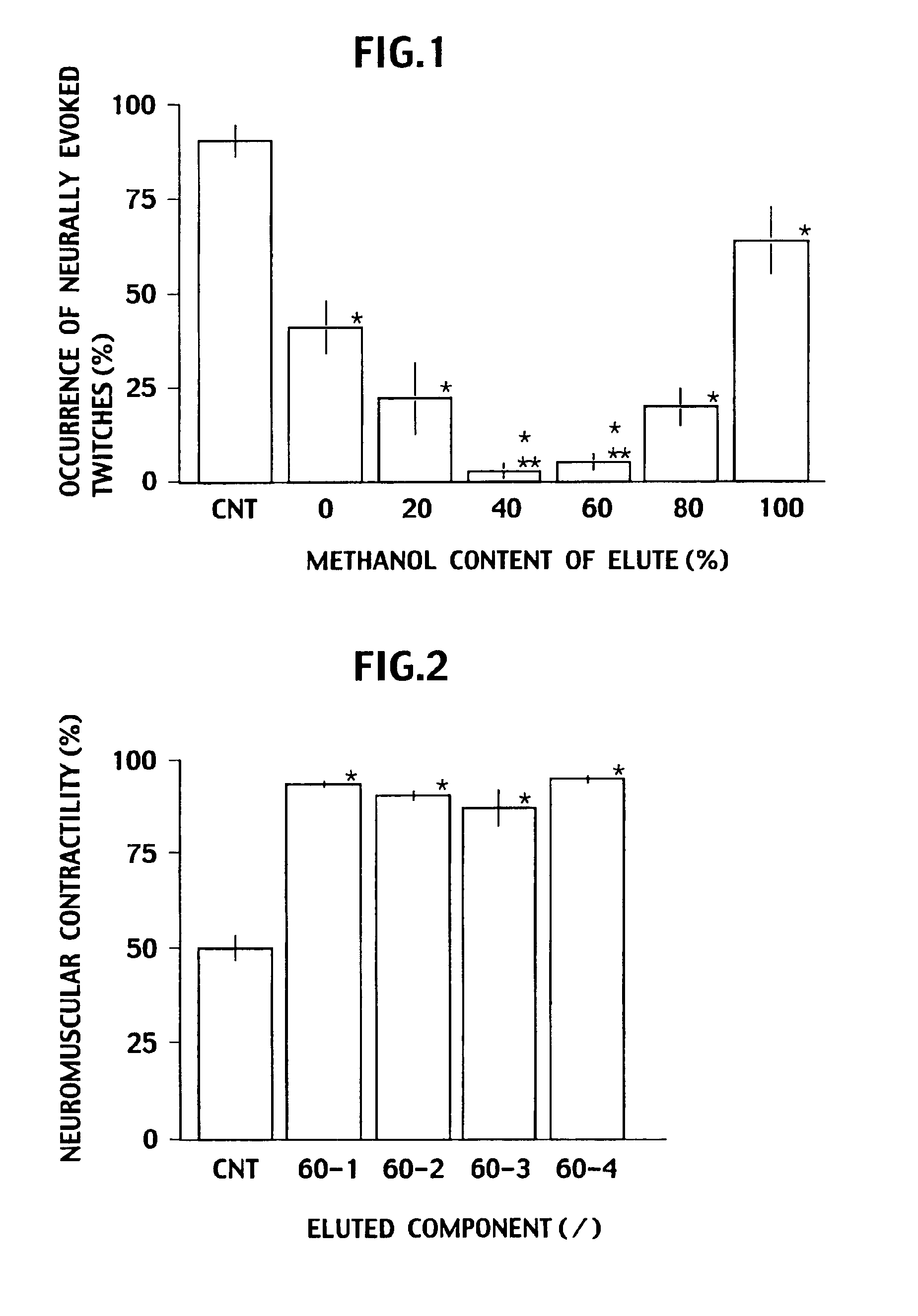
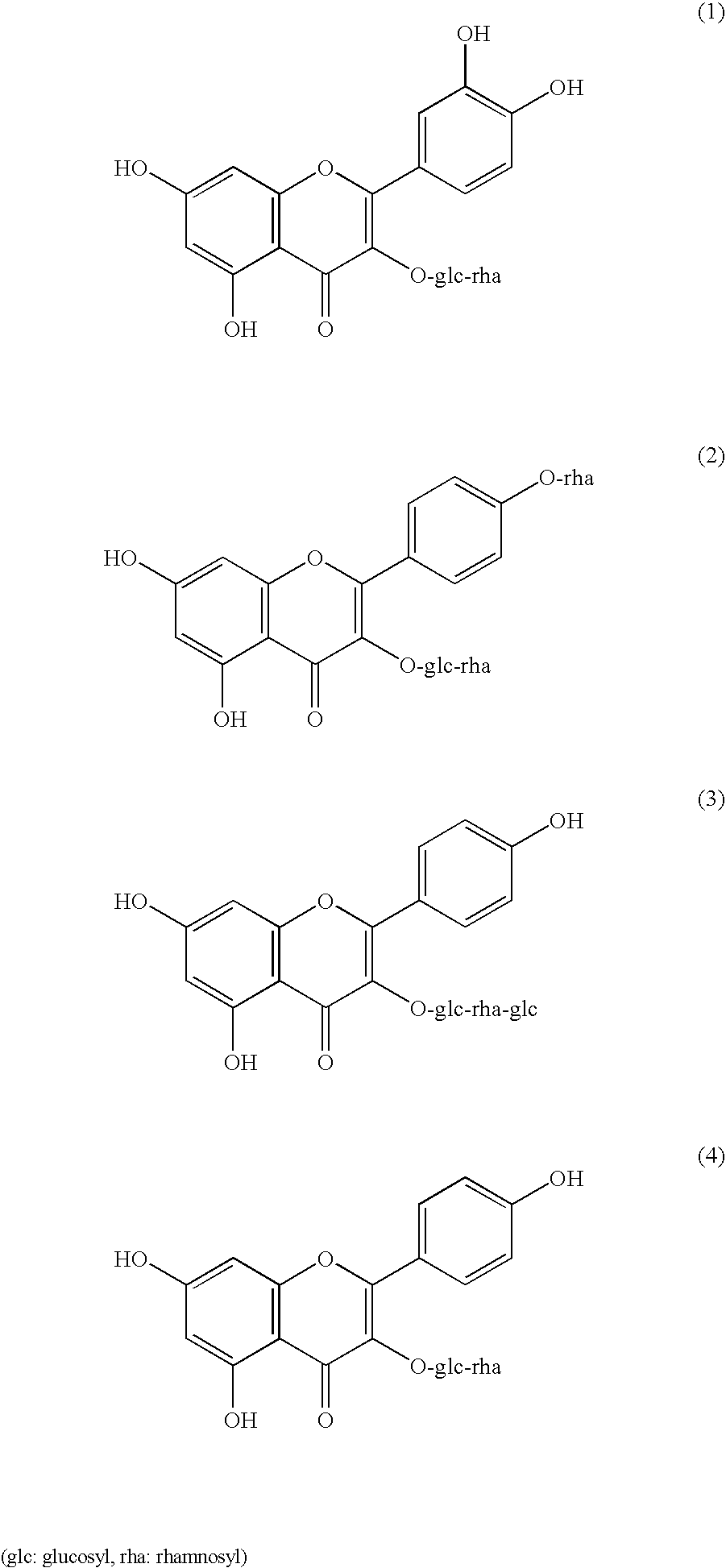
Disclosed is a neutralizing agent for a clostridium bacterial neurotoxin and a preparation method thereof. The neutralizing agent contains a flavonol glycoside (A) or a flavonol glycoside (B). The flavonol glycoside (A) has a flavone skelton which has two hydroxyl groups at the 5 and 7 positions, at least one hydroxyl group bonding at either one of the 3′, 4′ and 5′ positions, and an ether linkage forming glycoside at the 3 position with a carbohydrate chain containing a rutinose skelton. The flavonol glycoside (B) has also another ether linkage at the 4′ position with a carbohydrate chain containing rhamnose. The neutralizing agent is prepared by separating the thearubigin fraction of black tea extract by elution with methanol / water solvent using a reverse phase liquid chromatography. A fraction eluted with methanol / water solvent containing 40% methanol and a fraction eluted with methanol / water solvent containing 60% methanol have high neutralizing activity, and at least one of them is collected.
Owner:SHOKUHIN SANGYO HIGH SEP
Method for measuring content of kaempferol-3-O-rutinoside in radix tetrastigme medicinal material
PendingCN111505139AReduce dosageSmall amount of sampleComponent separationMedicinal herbsFluid phase
The invention provides a method for determining the content of kaempferol-3-O-rutinoside in a radix tetrastigme medicinal material, and belongs to the technical field of chemical detection. Accordingto the method, the content of kaempferol-3-O-rutinoside in the radix tetrastigme medicinal material is determined through high performance liquid chromatography, the sampling amount is small, the dosage of an extraction solvent is small, an extraction solution does not need to be concentrated, the determination method is simple, convenient, rapid and good in repeatability, and the quality of the radix tetrastigme medicinal material can be accurately evaluated. The result of the embodiment shows that the separation degree of the kaempferol-3-O-rutinoside peak and other peaks in the spectrogramobtained by the method is greater than 1.5, which shows that the separation degree is good; in a linear relation test, the linear relation of the sample size of the determination method disclosed by the invention is good in a range of 0.004-0.12 [mu] g; in a repeatability test, the relative standard deviation of the content of kaempferol-3-O-rutinoside in the determination method is 2.76%, which indicates that the method provided by the invention has good repeatability.
Owner:TONGDE HOSPITAL OF ZHEJIANG PROVINCE
Application of pelargonidin 3-O-rutinoside in preparation of drug for resisting serratia marcescens infection
ActiveCN111991409AAvoid infectionInhibition formationAntibacterial agentsOrganic active ingredientsPharmacologyPelargonidin
The invention discloses application of pelargonidin 3-O-rutinoside in preparation of a drug for resisting serratia marcescens infection. The invention discovers that the pelargonidin 3-O-rutinoside has the effect of inhibiting serratia marcescens infection for the first time, and does not inhibit the growth of serratia marcescens under an effective dose. The invention discovers that the combined medication of the pelargonidin 3-O-rutinoside and ciprofloxacin can effectively inhibit the formation of a serratia marcescens biofilm for the first time, and indicates that the pelargonidin 3-O-rutinoside has a good clinical application prospect in prevention and treatment of serratia marcescens infection.
Owner:XUZHOU UNIV OF TECH
Method for extracting myricetin-3-O-rutinose from taxus mairei leaves
ActiveCN103030673BSimple extraction methodEasy to implementSugar derivativesSugar derivatives preparationAlcoholPolyamide
The invention discloses a method for extracting myricetin-3-O-rutinose from taxus mairei leaves. The method comprises the steps of using a supercritical extraction kettle to extract, using methyl alcohol to absorb, using AB-8 resin to conduct chromatography, using 100-mesh polyamide to conduct the chromatography, collecting an eluent at 6-9min, concentrating and drying, and obtaining a white crystallized compound of myricetin-3-O-rutinose. The extracting method is simple and easy to implement, and the purity of myricetin-3-O-rutinose in the extracted white crystallized compound reaches 99.76%.
Owner:NINGBO INST OF TECH ZHEJIANG UNIV ZHEJIANG
Process for preparing kaempferol derivatives
InactiveCN100340569CIncrease contentCultivation is simpleSugar derivativesSugar derivatives preparationGlucose polymersD-Glucose
The invention provides a novel process for preparing Kacmpferol derivative, i.e. Kacmpferol-3-0-rutinose glycoside and Kacmpferol-3-0-glucose glycoside monomer or their mixture by using the white flowers or light-yellow flowers of Carthamus tinctorius L. as raw material, extracting with water or water-containing ethanol, and isolating and purifying through microporous resin and silica gel column chromatography.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Capsule for treating infantile autism and preparation method thereof
ActiveCN113318198ARegulatory compositionRegulatory activityNervous disorderUnknown materialsInfantile autismNervous system
The invention provides a capsule for treating infantile autism and a preparation method thereof. The capsule is prepared from the following raw materials in parts by weight: 33 to 62 parts of galactooligosaccharide, 12 to 28 parts of succinic anhydride, 20 to 33 parts of pumpkin polysaccharide, 22 to 40 parts of rutinose, 12 to 33 parts of rice fermentation filtrate, 12 to 25 parts of beta-carotene, 10 to 19 parts of strain, 10 to 20 parts of filler and 0.1 to 3 parts of adhesive. The raw materials are reasonably selected and scientifically proportioned, and all the components interact with one another to selectively stimulate the composition and activity of specific intestinal flora; and through a regulating system for regulating bidirectional signal communication between the intestinal tract and the nervous system, the composition and activity of the specific intestinal flora are selectively stimulated.
Owner:广东康盾高新技术产业集团股份公司
Method for simultaneously separating and preparing two petunidin anthocyanins in lycium ruthenicum
PendingCN114716493AUnique preparation methodSimple methodSugar derivativesSugar derivatives preparationBiochemistryPetunia
The invention relates to a preparation method of a lycium ruthenicum purified product, in particular to a method for simultaneously separating and preparing two petunidin anthocyanins in lycium ruthenicum. Purifying the crude extract by using macroporous resin, collecting acidic ethanol eluant, concentrating under reduced pressure to obtain anthocyanin macroporous resin eluant, further purifying the anthocyanin macroporous resin eluant by using a gel column, collecting the anthocyanin macroporous resin eluant in each 50mL / tube, collecting the fifth to seventh tubes and the 19th to 24th tubes, and respectively merging the eluant in sections to obtain the anthocyanin macroporous resin. Respectively carrying out vacuum concentration and freeze-drying on the two sections of eluents to obtain two petunidin anthocyanin, namely petunidin-3-O-rutinose (coumaroyl)-5-O-diglucoside and petunidin-3-O-rutinose (coumaroyl)-5-O-glucoside; the method is unique, two products with similar structures in the lycium ruthenicum can be separated at the same time, and an important theoretical basis is provided for qualitative and quantitative research of the anthocyanin of the lycium ruthenicum, structure-function relationship research of the anthocyanin of the lycium ruthenicum and standard substance development.
Owner:WOLFBERRY SCI INST NINGXIA ACAD OF AGRI & FORESTRY SCI
A method for extracting, separating and purifying four main anthocyanins from blackcurrant pomace
ActiveCN107325138BHigh yieldIndustrial simplicitySugar derivativesSugar derivatives preparationChromatographic separationGlycoside
The invention relates to a method for extracting, separating and purifying four main anthocyanins from the blackcurrant residue. The object is to provide a simple, economic, green, eco-friendly, and high-yield method for extracting, separating and purifying four main high-purity anthocyanins from the blackcurrant residue. According to the method, the blackcurrant waste residue produced from industrial production is taken as a raw material, and homogenized pulverized coupling enzyme hydrolysis technology, negative-pressure cavitation reinforced extraction technology, macroporous adsorption resin concentration technology, medium-pressure quick-flash reverse chromatographic separation technology and low-temperature crystallization and recrystallization technology are adopted to obtain four anthocyanin monomer ingredients including delphinidin 3-O-glucoside, delphinidin 3-O-rutinoside, cyanidin 3-O-glucoside, and cyanidin 3-O-rutinoside and having the purity reach 95%. The method is abundant and available in raw materials, short in time consumption, less in solvent consumption, high in purity of obtained anthocyanins, low in production cost, and suitable for massive industrial production.
Owner:GREATER KHINGAN RANGE CHAOYUE WILD BERRY DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com