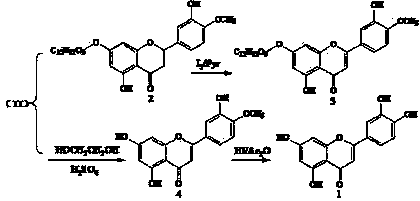
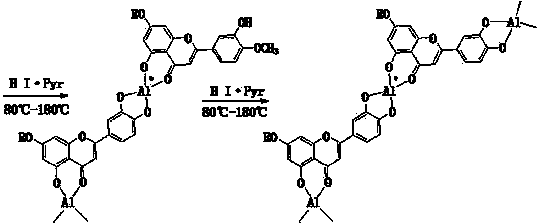
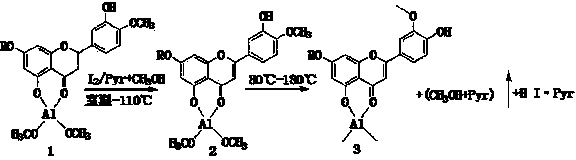
Semi-synthesis method of luteolin and galuteolin as well as luteolin rutinoside
A technology of luteolin and luteolin, which is applied in the field of chemistry and medicine, and can solve the problems of large environmental pollution, difficult recycling, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of Luteolin-7-O-Rutinoside from Hesperidin
[0044]Quickly weigh 3.5g of anhydrous aluminum chloride (AR) into a 250ml iodine measuring bottle, quickly add 15ml of pyridine (AR), shake well, add 8ml of methanol (AR), after 2min, add 16.5g of 92% hesperidin , stir for 2 minutes, add 6.8g of iodine (AR), add 7ml of methanol (AR), stir for 2 minutes, connect a condenser tube or an air condenser tube to the end of the iodine measuring bottle, and react in a closed manner at 80°C (to prevent the absorption of moisture in the air), every 1h Stir once, and form a homogeneous solution after 2 hours, and continue to seal the reaction for 6 hours (complete dehydrogenation). Distill in an air bath at 114°C for 15 hours (demethylation reaction, the bottle changes from solution to solid, soft when hot, hard when cooled), place at 80°C, add 10ml ethanol and 10ml glycerol, and Keep warm at 80°C for 2h, the solid turns into a solution, add 1.0g of hydrosulfite, ...
Embodiment 2
[0045] Example 2 Preparation of Luteolin-7-O-Rutinoside from Hesperidin by Acid Hydrolysis to Obtain Luteolin and Luteolin
[0046] Quickly weigh 3.5g of anhydrous aluminum chloride (AR) into a 250ml iodine measuring bottle, quickly add 15ml of pyridine (AR), shake well, add 8ml of methanol (AR), after 2min, add 16.5g of 92% hesperidin , stir for 5 minutes, add 6.8g of iodine (AR), add 7ml of methanol (AR), stir for 3 minutes, connect a condenser or air condenser to the end of the iodine measuring bottle, and react in a closed manner at 80°C (to prevent the absorption of moisture in the air), every 1h Stir once, and form a homogeneous solution after 2 hours, and continue to seal the reaction for 6 hours (complete dehydrogenation). Distill in an air bath at 112°C for 17h (demethylation reaction, the bottle changes from solution to solid, soft when hot, hard when cooled), place at 80°C, add 20ml of ethanol, and keep at 80°C for 3h, Turn the solid into a solution, add 1.0g of...
Embodiment 3
[0047] Example 3 Preparation of luteolin from hesperidin-7-O-rutinoside by acid-alcohol solution hydrolysis to obtain luteolin
[0048] Quickly weigh 3.5g of anhydrous aluminum chloride (AR) into a 250ml iodine measuring bottle, quickly add 15ml of pyridine (AR), shake well, add 8ml of methanol (AR), after 2min, add 16.5g of 92% hesperidin , stir for 5 minutes, add 6.8g of iodine (AR), add 7ml of methanol (AR), stir for 3 minutes, connect a condenser or air condenser to the end of the iodine measuring bottle, and react in a closed manner at 80°C (to prevent the absorption of moisture in the air), every 1h Stir once, and form a homogeneous solution after 2 hours, and continue to seal the reaction for 6 hours (complete dehydrogenation). Distill in an air bath at 116°C for 15h (demethylation reaction, the bottle changes from solution to solid, soft when hot, hard when cooled), place at 80°C, add 20ml of ethanol, and keep at 80°C for 3h, Turn the solid into a solution, add 1.0g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com