Application of kaempferol-3-O-rutinoside to preparation of medicine for treating PCSK9 (Proprotein convertase subtilisin/kexin type 9)-mediated disease
A technology of rutinoside and kaempferol, applied in the field of treating PCSK9-mediated diseases, can solve problems such as pain and allergies, poor compliance of PCSK9 monoclonal antibody injections, and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1, the influence of kaempferol-3-O-rutinoside on TG, TC, HDL-C and LDL-C of hyperlipidemia mice
[0020] 1. Experimental animals
[0021] Healthy female ICR mice, clean grade, weighing 18-22 g, were provided by the Animal Center of Yangzhou University. The experimental animals were raised in an independent environment with 12h-12h alternation of day and night, the room temperature was maintained at 24±2°C, water and food were free to drink, and the experiment was carried out after 1 week of adaptation to the environment. All handling of animals followed the requirements of the Ethics Committee of the International Association for the Study of Pain.
[0022] 2. Experimental materials
[0023] Kaempferol-3-O-rutinoside (Chengdu Ruifensi Biotechnology Co., Ltd.). Basic feed and high-fat feed (containing 3% cholesterol). Triglyceride (TG) test kits, total cholesterol (TC) test kits, low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein chol...
Embodiment 2
[0035] Embodiment 2, expression and purification of PCSK9 and EGF-A protein
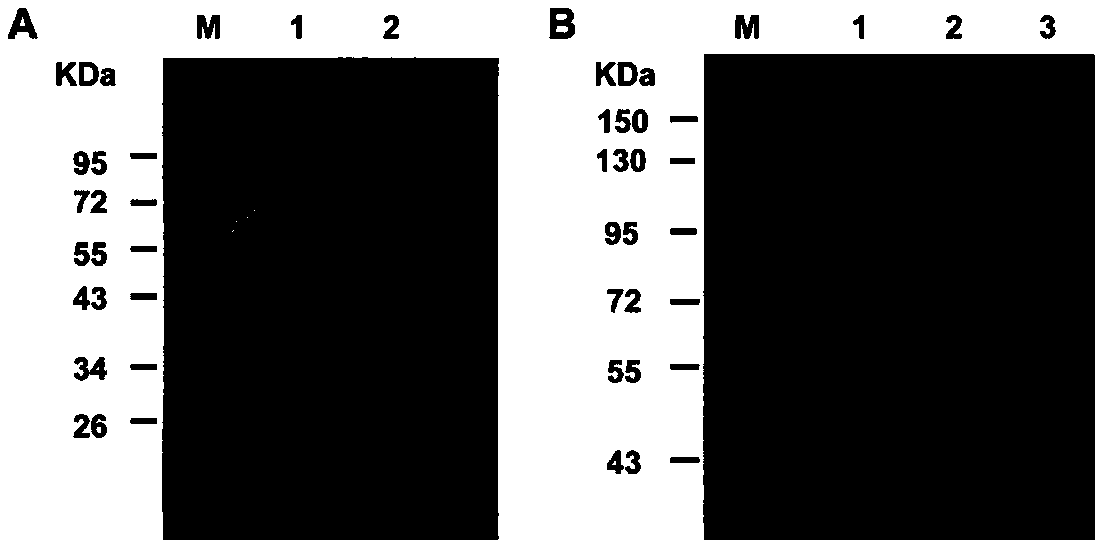
[0036]PCSK9 mainly binds to the EGF-A domain of LDLR, leading to the degradation of LDLR, reducing the ability of cells to absorb LDL, and exerting its activity in regulating lipid metabolism. In order to facilitate purification and subsequent detection, the expression and purification of PCSK9 and EGF-A proteins were performed separately. A His tag was added to the N-terminal of PCSK9, and a GST tag was added to the N-terminal of EGF-A. His-PCSK9 [12] and GST-EGF-A [13] The expression and purification of protein were carried out according to literature. His-PCSK9 crude bacterial solution was adsorbed by Ni column, and imidazole was eluted sequentially to obtain His-PCSK9 protein ( figure 1 ). The GST-EGF-A crude bacterial liquid is captured by the GST adsorption column, and glutathione is eluted to obtain the GST-EGF-A protein ( figure 2 ).
Embodiment 3
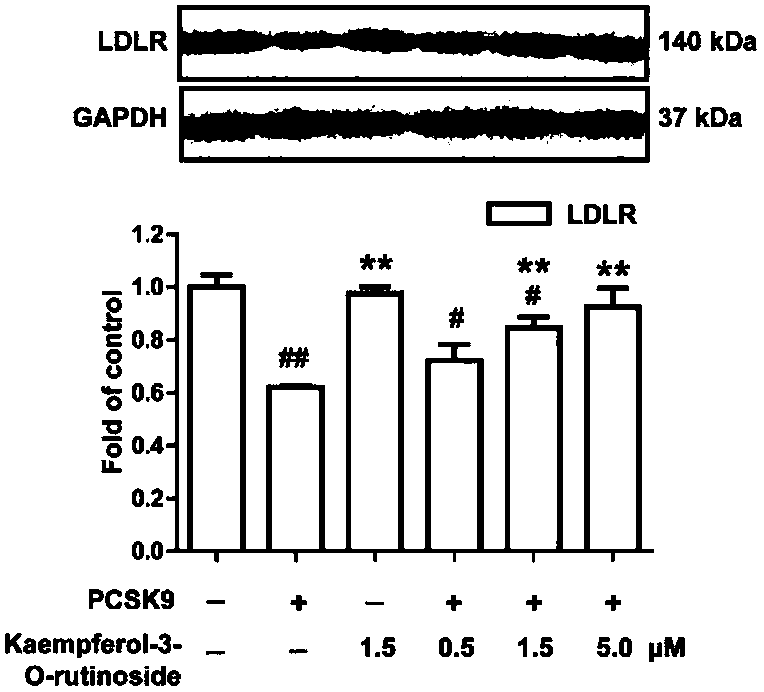
[0037] Example 3. Research on the mechanism of kaempferol-3-O-rutinoside inhibiting PCSK9
[0038] 3.1 PCSK9-induced LDLR degradation cell model and compound intervention
[0039] 3.1.1 Experimental materials
[0040] Kaempferol-3-O-rutinoside (Chengdu Ruifensi Biotechnology Co., Ltd.). Tris, TEMED, β-Mercaptoethanol, glycine, Tween-20, SDS, 30% Acrylamide / Bis, Ammonium Persulfate, GAPDH were purchased from sigma; Marker was purchased from Thermo; bromophenol blue, glycerin, Bovine SerumAlbumin were purchased from Sunshine; methanol, Sodium chloride and glycerol were purchased from Nanjing Chemical Reagent Co., Ltd.; LDLR antibody and anti-rabbit were purchased from cellsignaling; RIPA lysate was purchased from Beyotime Biotechnology; ECL luminescent liquid was purchased from Pierce.
[0041] HepG2 human liver cancer cells were purchased from ATCC, USA.
[0042] 3.1.2 Experimental grouping
[0043] Take HepG2 human liver cancer cells in the logarithmic growth phase, digest...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com