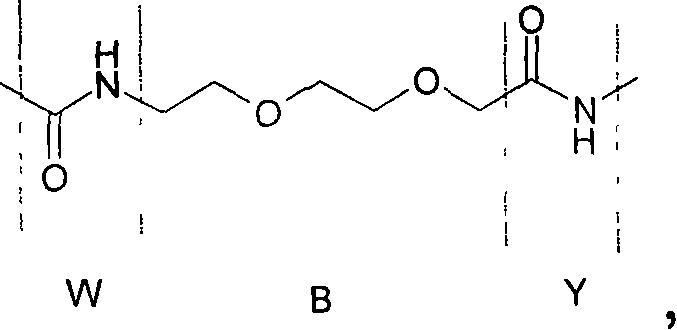
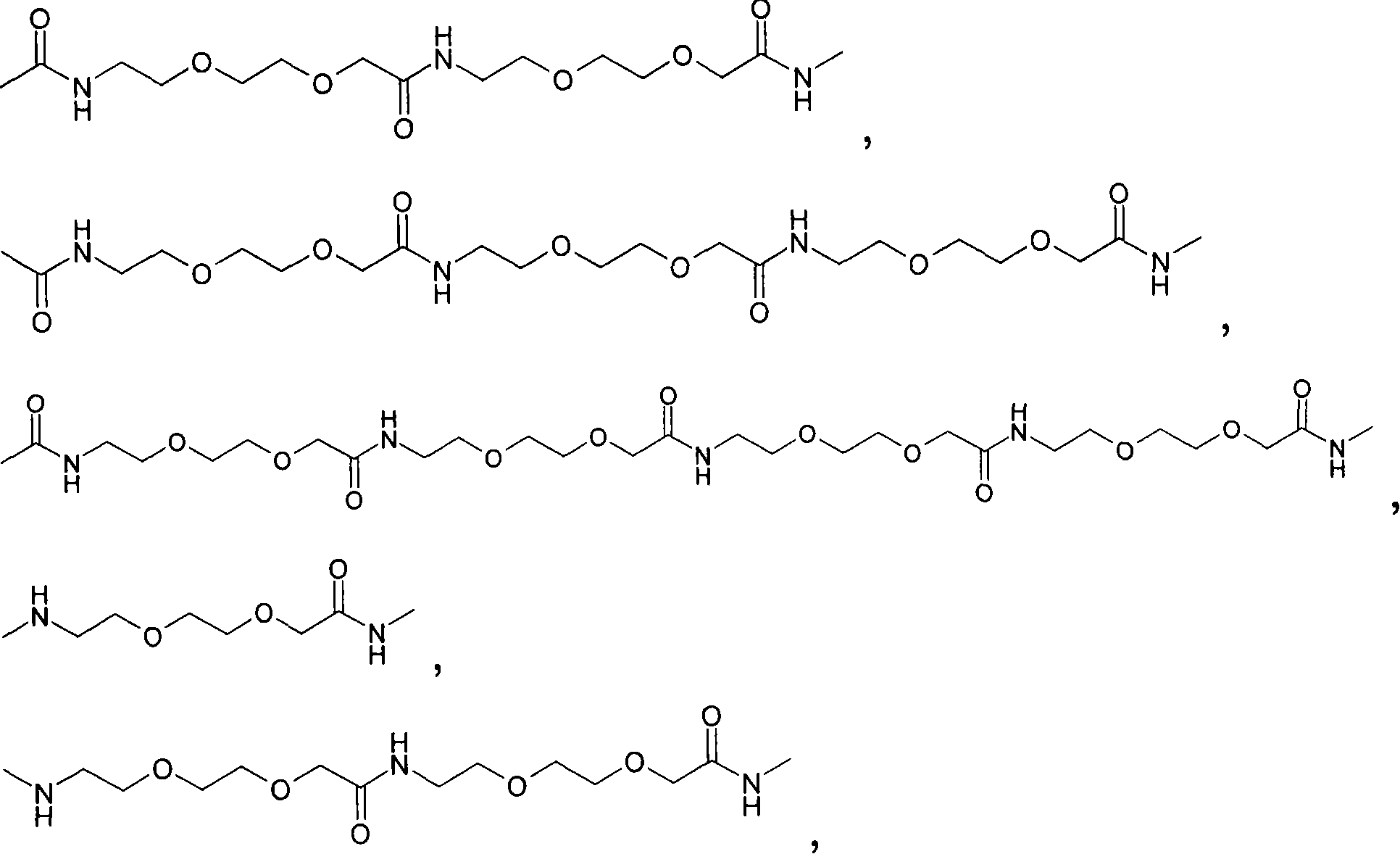
Novel glp-1 derivatives
A technology for albumin and compounds, applied in the field of albumin-binding derivatives of therapeutic peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0564] N ε37 -(2-(2-(2-(Dodecylamino)ethoxy)ethoxy)acetyl)-[Aib 8,22,35 Lys 37 ]GLP-1(7-37)amide
[0565]
[0566] The initial sequence was generated using the resin (Rink amide, 0.68 mmol / g, Novabiochem, 0.25 mmole) on an ABI433A machine following the manufacturer's instructions. All protecting groups are acid labile with the exception of the residue used at position 37 (Fmoc-Lys(ivDde)-OH, Novabiochem), allowing specificity for this lysine but not any other lysine. off protection.
[0567] process
[0568] The resin (0.25 mmole) containing the GLP-1 analog amino acid sequence prepared above was placed in a manual shaker / filter device, and was treated with 2% hydrazine dissolved in N-methylpyrrolidone (2×20ml, 2×12min ) to remove the DDE group, followed by washing with N-methylpyrrolidone (4×20 ml). Fmoc-8-amino-3,6-dioxoctanoic acid (Neosystem FA03202) (4 molar equivalents relative to the resin) was dissolved in N-methylpyrrolidone / dichloromethane (1:1, 20 ml). Hyd...
Embodiment 2
[0573] N ε37 -(2-(2-(2-(17-Sulfohexadecanoylamino)ethoxy)ethoxy)acetyl)-[Aib 8,22,35 , Lys 37 ]GLP-1(7-37)amide
[0574]
[0575] Compounds were prepared according to the methods in Example 1 and "General Synthetic Methods".
[0576] HPLC: (Method A1): RT=45.5min
[0577] LCMS: m / z=792.9 (M+5H) 5+ ,990.9(M+4H) 4+ , 1320.9(M+3H) 3+ , Calculated (M+H) + =3959.9
Embodiment 3
[0579] N ε37 -{2-[2-(2-(15-Carboxypentadecanoylamino)ethoxy)ethoxy]acetyl}-[Aib 8,22,35 , Lys 37 ]GLP-1(7-37)amide
[0580]
[0581] Compounds were prepared according to the methods in Example 1 and "General Synthetic Methods".
[0582] HPLC: (Method B1): RT=43.8min
[0583] HPLC: (Method A1): RT=42.0min
[0584] LCMS: m / z=978.3 (M+4H) 4+ , 1303.8(M+3H) 3+ , Calculated (M+H) + =3909.6
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com