Method for producing folic acid antagonist and its intermediate
A Chinese-style, metal catalyst technology, applied in organic chemistry and other fields, can solve problems affecting the purity of finished products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
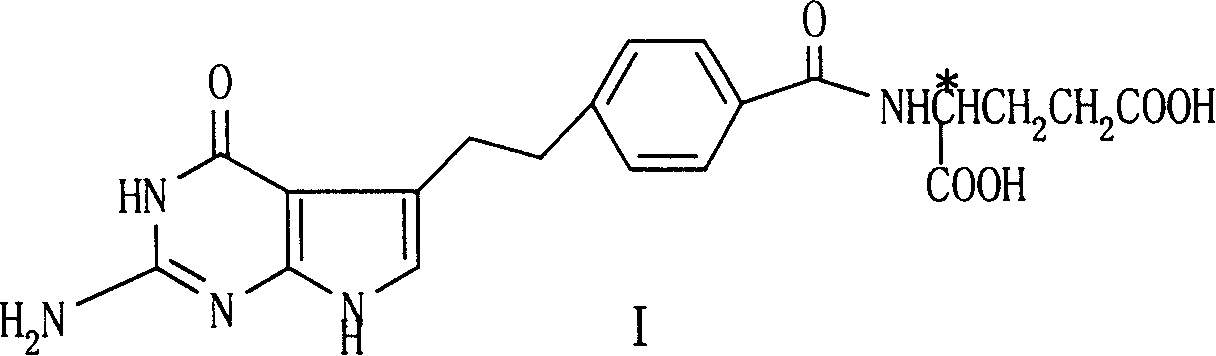
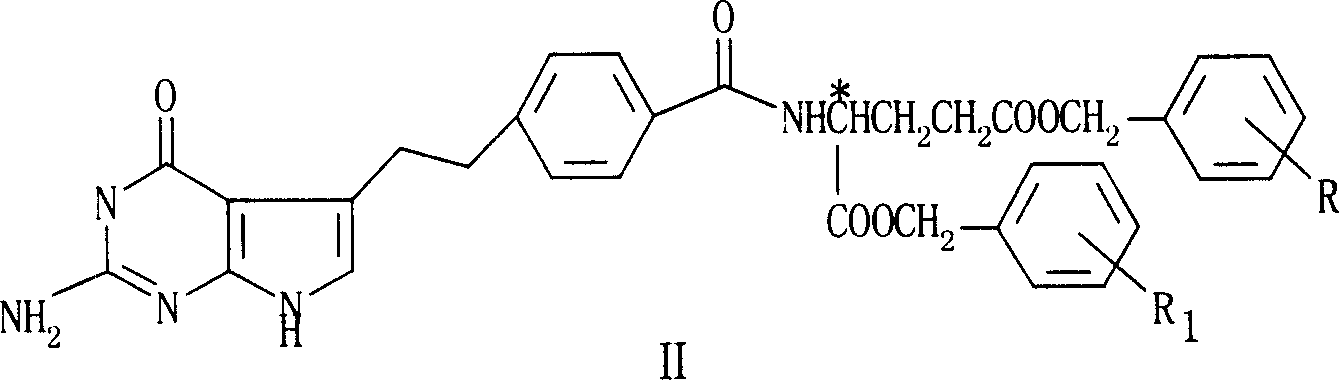
[0028] N-(4-[2-(2-Amino-4(3H)-oxy-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl-L-glutamic acid di Benzyl ester
[0029] 10 grams (33.6 mmol) of 4-[2-(2-amino-4(3H)-oxygen-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoic acid and 100ml dimethyl Put methyl formamide into a 250ml three-necked flask, stir, cool to 5°C, add 10ml (91mmol) of N-methylmorpholine, 7.5g (42.7mmol) of 2-chloro-4,6-dimethoxytriazine, Stir at 5-10°C for 1 hour, add 20 g (40.2 mmol) of L-dibenzyl glutamate p-toluenesulfonate, stir at 25°C for 2 hours, pour into a 500 ml separating funnel, add 100 ml of dichloromethane 100ml of water, shake well, separate the organic layer, extract the aqueous layer with dichloromethane, combine the organic layers, evaporate the organic solvent under reduced pressure to the utmost, add 50ml of ethyl acetate and stir, filter, wash with ethyl acetate successively, After drying, 15 g of off-white solid was obtained, with a yield of 73.5%.
[0030] 1 HNMR (400MHz DMSO d 6 )δ ...
Embodiment 2
[0036] N-(4-[2-(2-Amino-4(3H)-oxy-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl-L-glutamic acid di Benzyl ester
[0037] 10 grams (15.2 mmol), 50ml (100mmol) of 2N sodium hydroxide were put into a 100ml reaction bottle, stirred at room temperature for 2 hours, and the reaction solution was added dropwise to 100ml of 4N sulfuric acid at 0°C. 60ml×2 extraction, combined, washed with 60ml of water, dried over anhydrous magnesium sulfate, filtered, the filtrate was evaporated to dryness under reduced pressure, added 30ml of ethyl acetate, stirred, filtered, washed with ethyl acetate, dried to obtain 7 grams of white solid, yield 75.9 %.
Embodiment 3
[0039] N-(4-[2-(2-amino-4(3H)-oxy-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl-L-glutamic acid
[0040] Put N-(4-[2-(2-amino-4(3H)-oxygen-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl- 5 g (8.2 mmol) of L-dibenzyl glutamate, 100 ml of methanol, 200 ml of tetrahydrofuran, stir to dissolve, add 1 g of 10% Pd / C, hydrogenate at room temperature and normal pressure for 3 hours, filter, wash with 50 ml of tetrahydrofuran, and depressurize The solvent was evaporated to give the title compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com