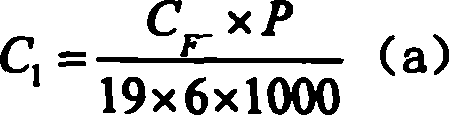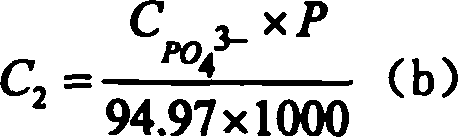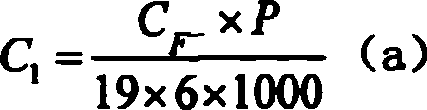Ion chromatography method for measuring lithium salt density in lithiumion cell electrolyte
A lithium ion battery and ion chromatography technology is applied in the field of determining the concentration of salts in the battery electrolyte, which can solve the problems of inconvenient methods, large errors and high equipment requirements, and achieve the effects of stable results and high accuracy.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Take NaF (Aldrich product) 2.210g, KH respectively 2 PO 4 (Aldrich product) 1.433g, diluted with secondary deionized water (18.2MΩ.cm) to a constant volume of 1L, and then diluted with secondary deionized water to concentrations of 20mg / L, 30mg / L, and 40mg / L , 100mg / L standard solution.
[0018] (2) Test above-mentioned standard solution with ion chromatography, make C-A standard curve according to concentration C and peak area response value A, its regression equation and linear coefficient are as shown in table 1.
[0019] anion
[0020] (3) Take 10mL of lithium-ion battery electrolytes with two known lithium salt concentrations (A: 1.12mol / L; B: 1.15mol / L respectively), then add 10mL of reverse aqua regia respectively, and let it stand for 12h .
[0021] (4) The solution after the above reaction is diluted step by step (100 times × 10 times × 2 times), and sent to ion chromatography to test the F - and PO 4 3- Concentration C F- and C PO43- , an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com