Recombinant adeno-associated virus comprising VEGFR Truncated Soluble cDNA and gene therapeutic agent comprising the same
A VEGFR-1, VEGFR-2 technology, applied in the field of recombinant adeno-associated virus containing Truncated Soluble cDNA of VEGFR and gene therapy agents containing the above components, can solve the problems such as no major breakthroughs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: Cloning of pAAV plasmids containing genes that inhibit neovascularization
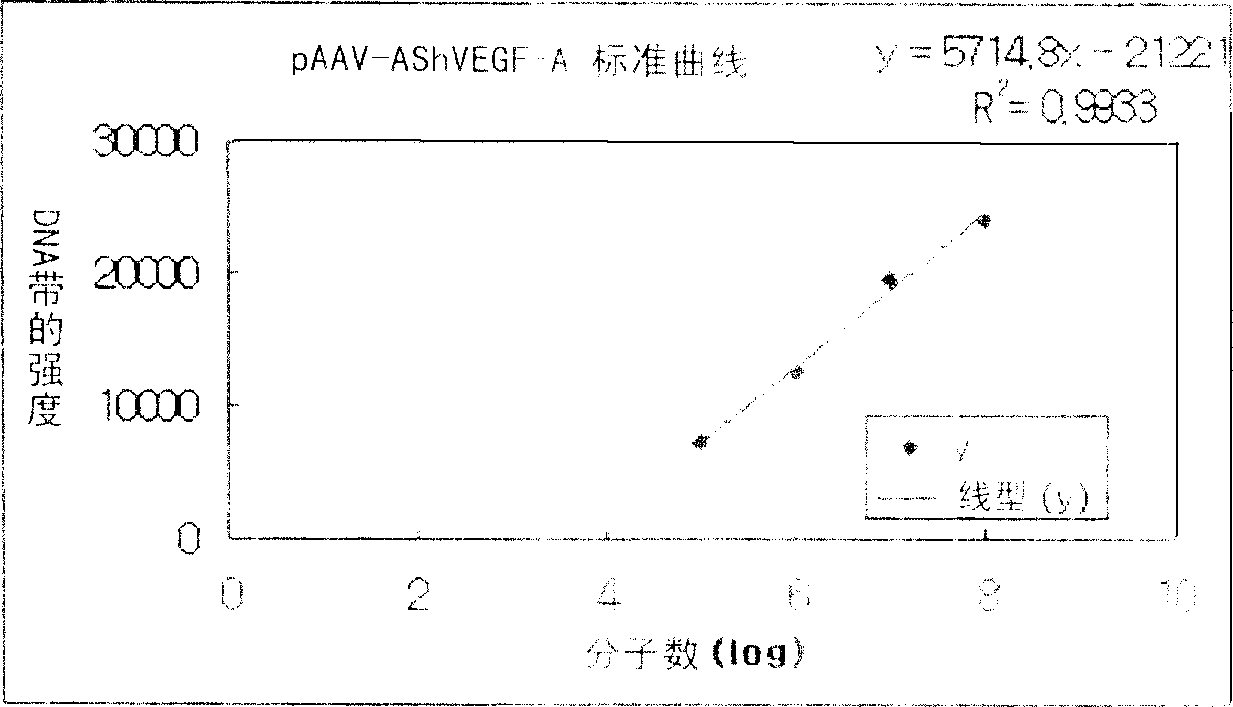
[0046] In order to use pAAV-FIX cis plasmad DNA (US 6,093,292) to insert VEGF-A (NCBI accession #NM_003376 for human) cDNA into pAAV-FIX cis plasmad DNA in the antisense direction according to the cloning method, use VEGF-A isoform antisense Trans-gene constructs of pAAV-AShVEGF-A were produced by replacing the factor IX cDNA gene contained in pAAV-FIX cis plasmad DNA with the sense cDNA. In addition, trans-gene constructs of pAAV-AShVEGF-A-IRES-EGFP in which VEGF-Aisoform antisense cDNA was linked to IRES-EGFP were prepared.
[0047] The truncated soluble cDNAs of VEGFR-1 (Flt-1; NCBIaccession#NM_002019 for human) and VEGFR-2 (Kdr / Flk-1; NCBIaccession#AF06365 8 for human), which act as receptors that interact with the three isoforms of VEGF, were also used The same method was used for insertion or replacement to produce trans-gene constructs of pAAV-TShVEGFR-1 and pAAV-TShVEGFR-2. ...
Embodiment 2
[0085] Embodiment 2: Making the rAAV vector of gene therapy agent for inhibiting neovascularization
[0086] In order to make a recombinant AAV (rAAV) vector for gene therapy, in addition to each pAAV plasmid DNA made in Example 1, the AAV rep-cap plasmid DNA (pAAV-RC plasmid) expressing the AAV rep part and cap part is also needed. , Stratagene Co., USA) and adenovirus helper plasmid (pHelper plasmid, Stratagene Co., USA). After all the above three plasmid DNAs were transfected into HEK293 (human embryonic kidney 293; ATCCCRL-1573) cells and cultured for 96 hours, the HEK293 cells were collected and ultrasonically disrupted, and the recombinant AAV (rAAV) particles were subjected to CsCl density gradient centrifugation for three times. , and then collect the fraction with RI (Refractive Index) of 1.37-1.41 g / ml for purification and separation.
[0087] The titration method of the above-mentioned isolated rAAV particles is as follows: PCR primer [CMV F1 (SEQ ID NO: 14): 5′-GG...
Embodiment 3
[0113] Embodiment 3: The treatment of cancer cell line culture and rAAV vector gene therapy agent
[0114] In order to study the anticancer effect of preventing cancer metastasis in vitro, human colorectal cancer cell line (T84: ATCC CCL-248) and human lung cancer cell line (LCSC#1: WO 02 / 061069) and human gastric cancer cell lines (NUGC3, MKN45, MKN74) and other five cancer cell lines. In order to test the anti-cancer therapeutic effect of recombinant rAAV on the above-mentioned cancer cell lines, each branch of the above-mentioned cancer cell lines on 6well plate 10 5 After cells / well cultured for 24 hours, add a total M.O.I. of 10 5 Various combinations of neovascularization-inhibiting rAAV vectors were cultured for 24 hr. In this example, the negative control (negative control) selects the same cell population that has not been treated with rAAV and the rAAV vector (rAAV-EGFP) that does not insert VEGF antisense and only expresses GFP, and is the same as the recombinant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com