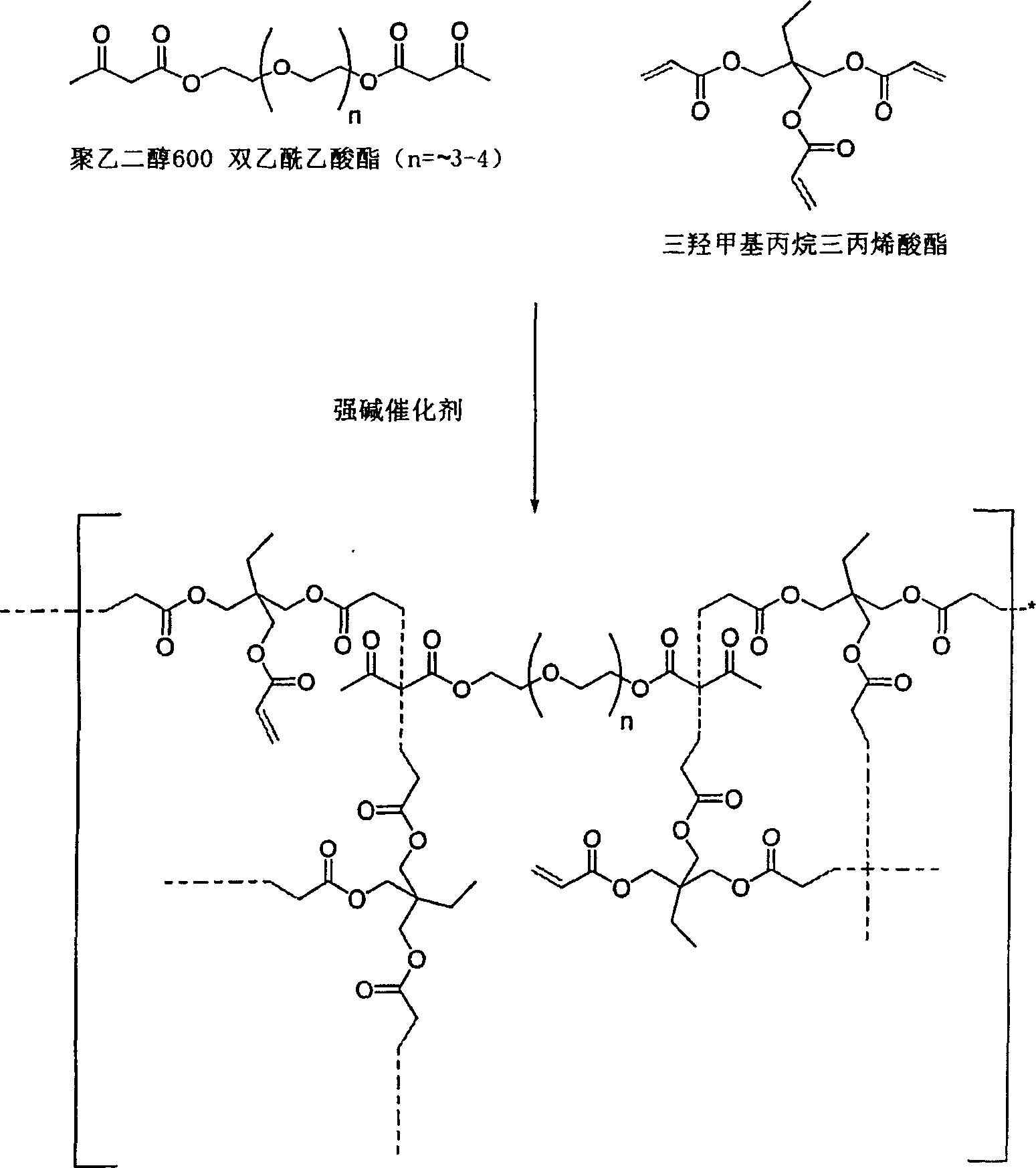
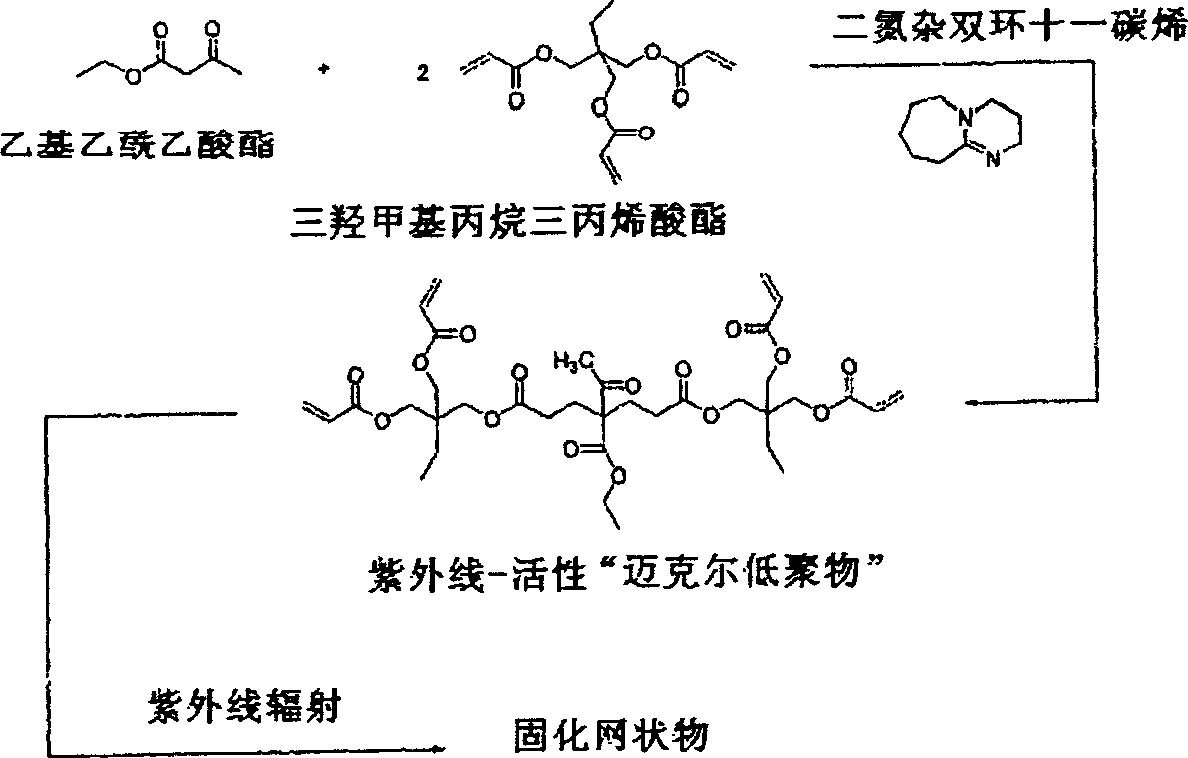
Dual cure reaction products of self-photoinitiating multifunctional acrylates with cycloaliphatic epoxy compounds
An epoxide, reaction product technology, applied in dentistry and other directions, can solve the problems of low adhesion and limitation of metal substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Novel Michael addition polyacrylate resins were synthesized based on the Michael donor ethyl acetoacetate and 2,4-pentanedione according to the methods described in US Patent 5945489 and US Patent 6025410. The Michael polyacrylate resins were blended with various cycloaliphatic epoxies at varying levels. The resin / epoxide composition was applied to a phosphated steel substrate and cured at a UV dose of 1500 mJ / cm2. All tests were carried out on cured resin coatings 24 hours after UV radiation to ensure that the subsequent so-called dark cure of the epoxy component was complete. The results are shown in Table I, Table II and Table III.
[0067] 1
2
3
4
5
Michael plus polypropylene
100.00
50.00
0.00
50.00
0.00
UVR 6105
0.00
50.00
100.00
0.00
0.00
UVR 6128
0.00
0.00
0.00
50.00
100.00
Sarto...
Embodiment 2
[0075] According to the method described in US Patent 5945489 and US Patent 6025410, Michael addition polyacrylate resin was synthesized based on 75:25 HDDA and TMPTA and ethyl acetoacetate. The resin with Uvacure TM 1562 (UCB Chemicals) Blend, Uvacure TM 1562 (UCB Chemicals) is an acrylate-cycloaliphatic epoxy blend containing two acrylate and epoxy functionalities. The mixture was applied to a phosphate steel or aluminum substrate and cured at a UV dose of 1500 mJ / cm2. Adhesion, solvent resistance, pencil hardness, gloss, abrasion resistance are as given above in the preceding examples. As explained in Example 1, all tests were performed 24 hours after UV irradiation.
[0076] 1
2
3
4
5
resin
Example 2
Example 2
Example 2
Example 2
---
---
1562
1562
1562
1562
weight % epoxy
---
10
25
50
100
adhesiveness
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com