Process for producing benzopyrone compound
A technology of compounds and compositions, applied in the field of preparation of benzopyrone compounds, capable of solving problems such as poor positional selectivity
Inactive Publication Date: 2010-09-15
SUMITOMO CHEM CO LTD
View PDF4 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, the site selectivity for the introduced nitro group during nitration is poor, and as a result, isomeric by-products with nitro groups at different substitution positions are generated
Therefore, a step for removing the isomer is required, and thus, the process involving the use of nitro-substituted 2-hydroxyacetophenone as a starting material is not necessarily a satisfactory industrial process
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
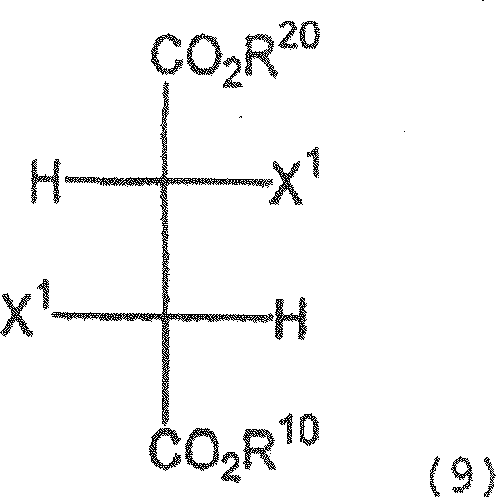
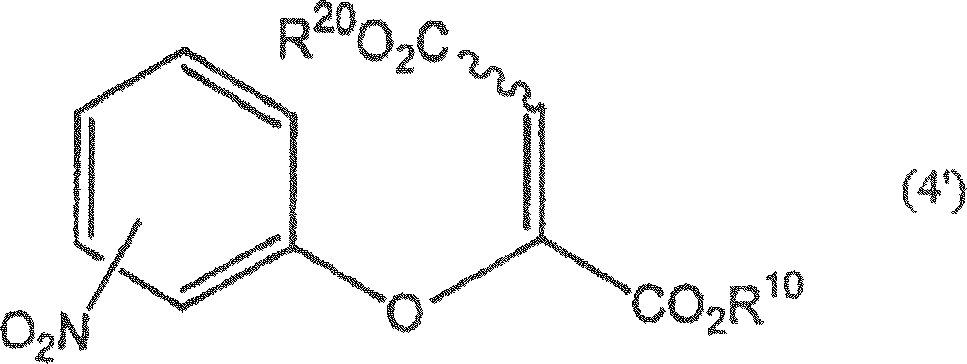
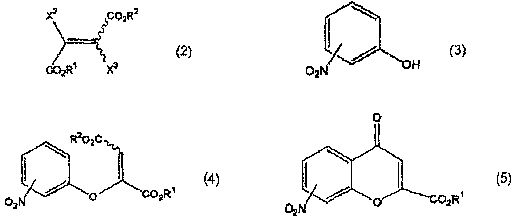
A process for producing a dicarboxylic acid compound represented by the formula (4): (4) (wherein R<1> and R<2> are the same or different and each represents lower alkyl; and the wavy line indicates that this compound is the (E) or (Z) isomer or a mixture of both), characterized by reacting a compound represented by the formula (2): (2) (wherein R<1>, R<2>, and the wavy line have the same meanings as the above; and one of X<2> and X<3> represents hydrogen and the other represents halogeno) with a nitrophenol represented by the formula (3) in the presence of a base; a process for producing a nitrochromone compound represented by the formula (5): (5) (wherein R<1> has the same meaning as the above), characterized by reacting the dicarboxylic acid compound or a carboxylic acid thereof with an acid; a process for producing an aminochromone compound which comprises reducing the nitrochromone compound; and a process for producing an amidochromone compound which comprises acylating the aminochromone compound. The method includes acylating aminochromone compound.
Description
This application is a divisional application of an application with a filing date of February 27, 2003, an application number of 03809174.7, and an invention title of "Preparation Method for Benzopyrone Compounds". technical field The invention relates to a preparation method of a benzopyrone compound, and the benzopyrone compound is a compound that can be used as a drug intermediate. Background technique The aminobenzopyrone compound is a compound useful as a drug intermediate (for example, Eur. J. Med. Chem., 32, 547 (1997), JP-A 3-95144, etc.), which is used in the preparation of the compound A known method involves reacting a nitrobenzopyrone compound with hydrogen in the presence of a palladium catalyst (for example, J. Chem. Soc. (C), 2230 (1970) etc.). However, the method is unsatisfactory as an industrial production method because the method tends to produce excessively reduced compounds in which the carbon-carbon double bond at the 2-position and / or the 4-position...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07C205/37C07C201/12C07C55/02C07C51/363C07D311/24C07C67/307C07C69/34
Inventor 日比野裕明大塚晋宫本泰延吉田大泰奥本五夫
Owner SUMITOMO CHEM CO LTD
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com