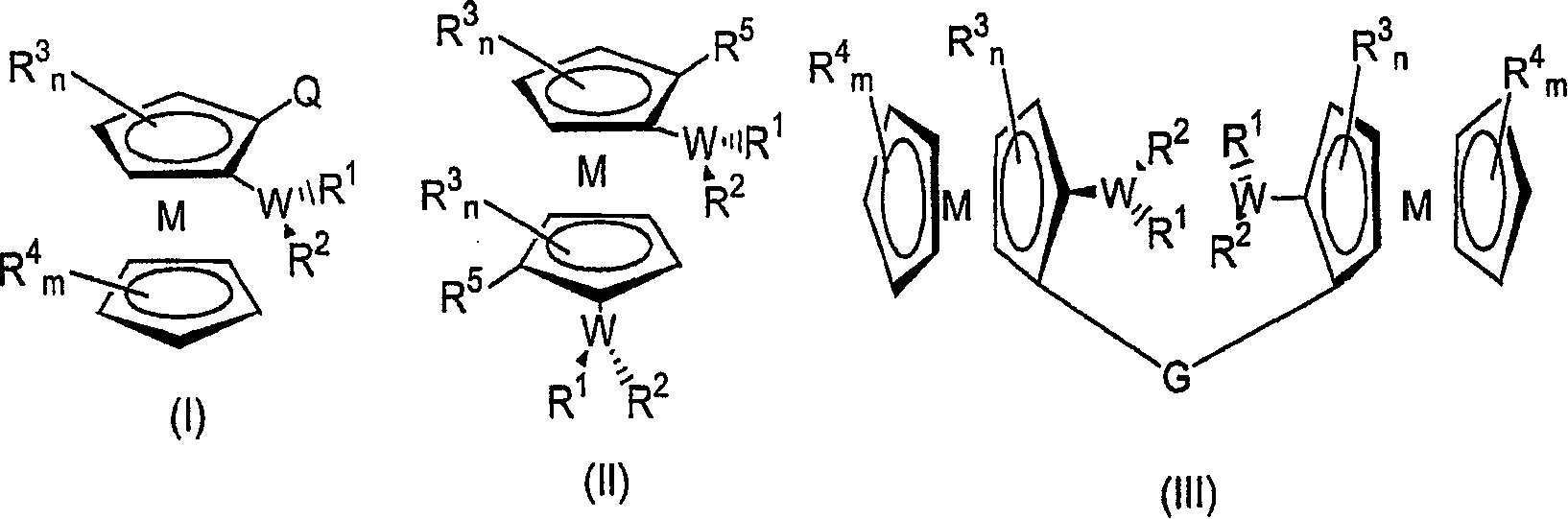
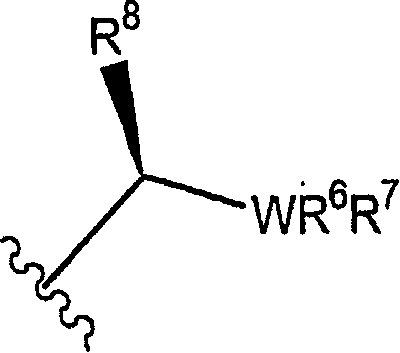
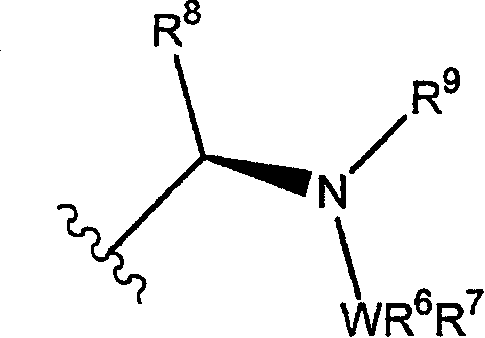
Metallocene-based chiral phosphine or arsine ligands
A ligand, metallocene technology, applied in the field of metal ligand complexes, to achieve the effect of a large selection range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] (R c , S Fe , S P )-2-[(1-N, N-dimethylamino)ethyl]-1-[(2-methoxyphenyl)phenylphosphino]ferrocene[(R c , S Fe , S p )-2]:
[0150]
[0151]Et 2 To a solution of O (50 mL) was added 1.7M t-BuLi in pentane (9.7 mL, 16.5 mmol) over 10 minutes. After complete addition, the mixture was warmed to room temperature and stirred at room temperature for 1.5 hours. The resulting red solution was recooled to -78°C, followed by the addition of dichlorophenylphosphine (2.24 mL, 16.5 mmol) in one portion. After stirring at -78°C for 10 minutes, the mixture was slowly warmed to room temperature and stirred at room temperature for 1.5 hours. The mixture was recooled to -78°C, followed by the slow addition of (2-methoxy)phenyllithium solution [from 2-bromomethoxybenzene (3.32 g, 17.7 mmol) and 1.7 M t-BuLi in pentane Solution (20.8mL, 35.4mmol) in Et 2 O (90mL), prepared by reaction at -78°C]. The mixture was warmed to room temperature overnight, then filtered through a pad ...
Embodiment 2
[0153] (R c , S Fe , S P )-2-[(1-N, N-dimethylamino)ethyl]-1-[(1-naphthyl)phenylphosphino]ferrocene[(R c , S Fe , S p )-3]:
[0154]
[0155] Et 2 To a solution of O (60 mL) was added 1.7M t-BuLi in pentane (12.94 mL, 22 mmol) over 10 minutes. After complete addition, the mixture was warmed to room temperature and stirred at room temperature for 1.5 hours. The resulting red solution was recooled to -78°C, then dichlorophenylphosphine (2.99 mL, 22 mmol) was added in one portion. After stirring at -78°C for 10 minutes, the mixture was slowly warmed to room temperature and stirred at room temperature for 1.5 hours. The mixture was recooled to -78 °C, followed by the slow addition of 1-naphthalenelithium solution [from 1-bromonaphthalene (5.38 g, 26 mmol) and 1.7M t-BuLi in pentane (30.6 mL, 52 mmol) in Et 2 O (120mL), prepared by reaction at -78°C]. The mixture was warmed to room temperature overnight, then filtered through a pad of Celite. The filtrate was concentr...
Embodiment 3
[0157] (R c , S Fe , S P )-2-[(1-N, N-dimethylamino)ethyl]-1-[(1-naphthyl)phenylphosphino]ferrocene[(R c , S Fe , S p )-3] and (R c , S Fe , R P )-2-[(1-N, N-dimethylamino)ethyl]-1-[(1-naphthyl)phenylphosphino]ferrocene[(R c , S Fe , R p )-4]:
[0158]
[0159] Et 2 To a solution of O (15 mL) was added 1.7M t-BuLi in pentane (3.2 mL, 5.5 mmol) over 10 minutes. After complete addition, the mixture was warmed to room temperature and stirred at room temperature for 1.5 hours. The resulting red solution was recooled to -78°C, then dichlorophenylphosphine (0.75 mL, 5.5 mmol) was added in one portion. After stirring at -78°C for 10 minutes, the mixture was slowly warmed to room temperature and stirred at room temperature for 1.5 hours. 1-Naphthyllithium solution [from 1-bromonaphthalene (1.35 g, 6.5 mmol) and 1.7M t-BuLi in pentane (7.6 mL, 13 mmol) in Et 2 O (30mL), prepared by reaction at -78°C]. The mixture was stirred overnight at room temperature, then filter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com