Donor type hemopoietic chimera and its prepn process
A chimera and artificial blood technology, applied in the field of biological cells, can solve the problem that human/animal chimeras cannot be used as a source of human blood cells, and achieve the effect of solving the problem of blood-borne diseases, avoiding complex requirements and high safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
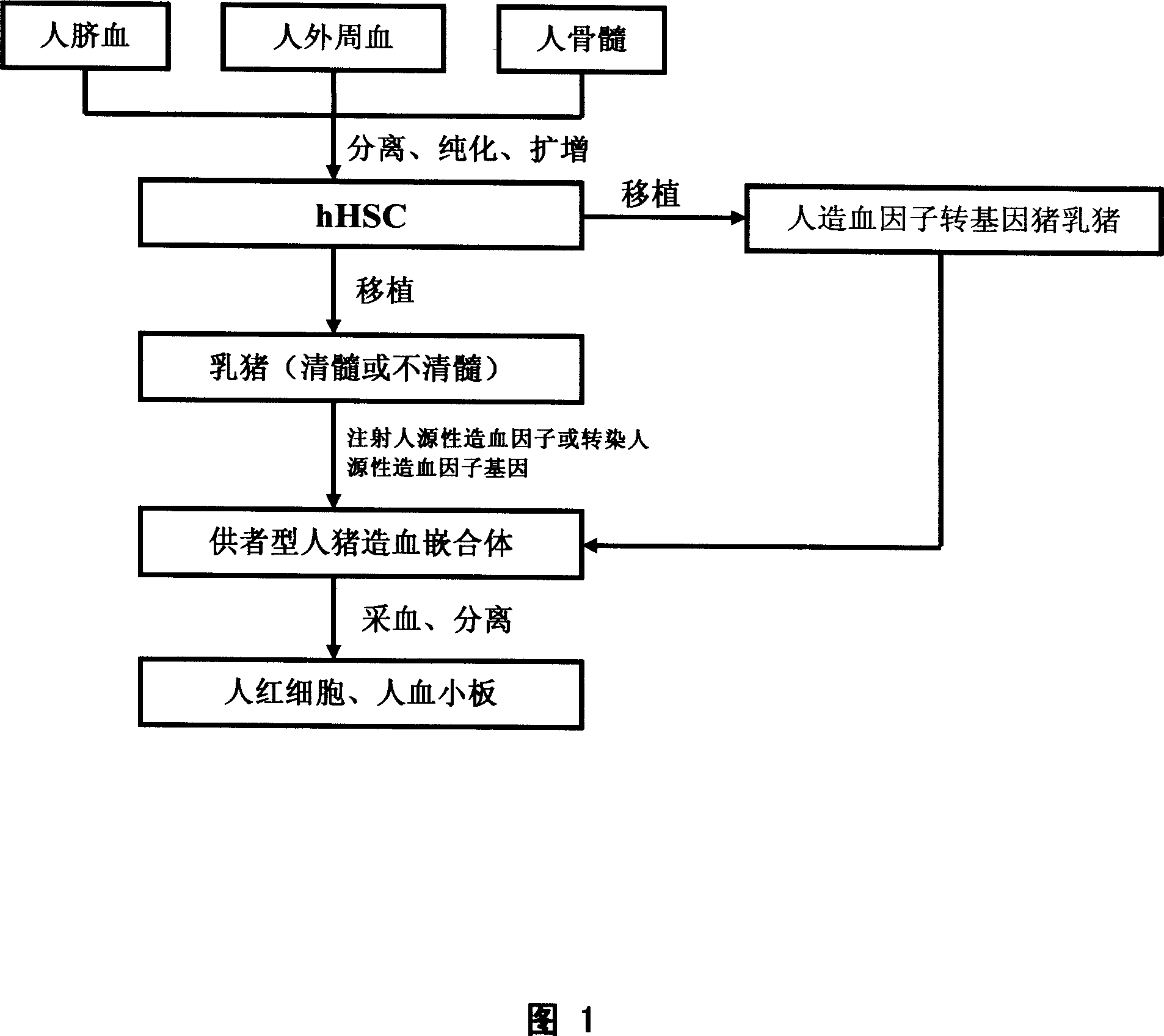
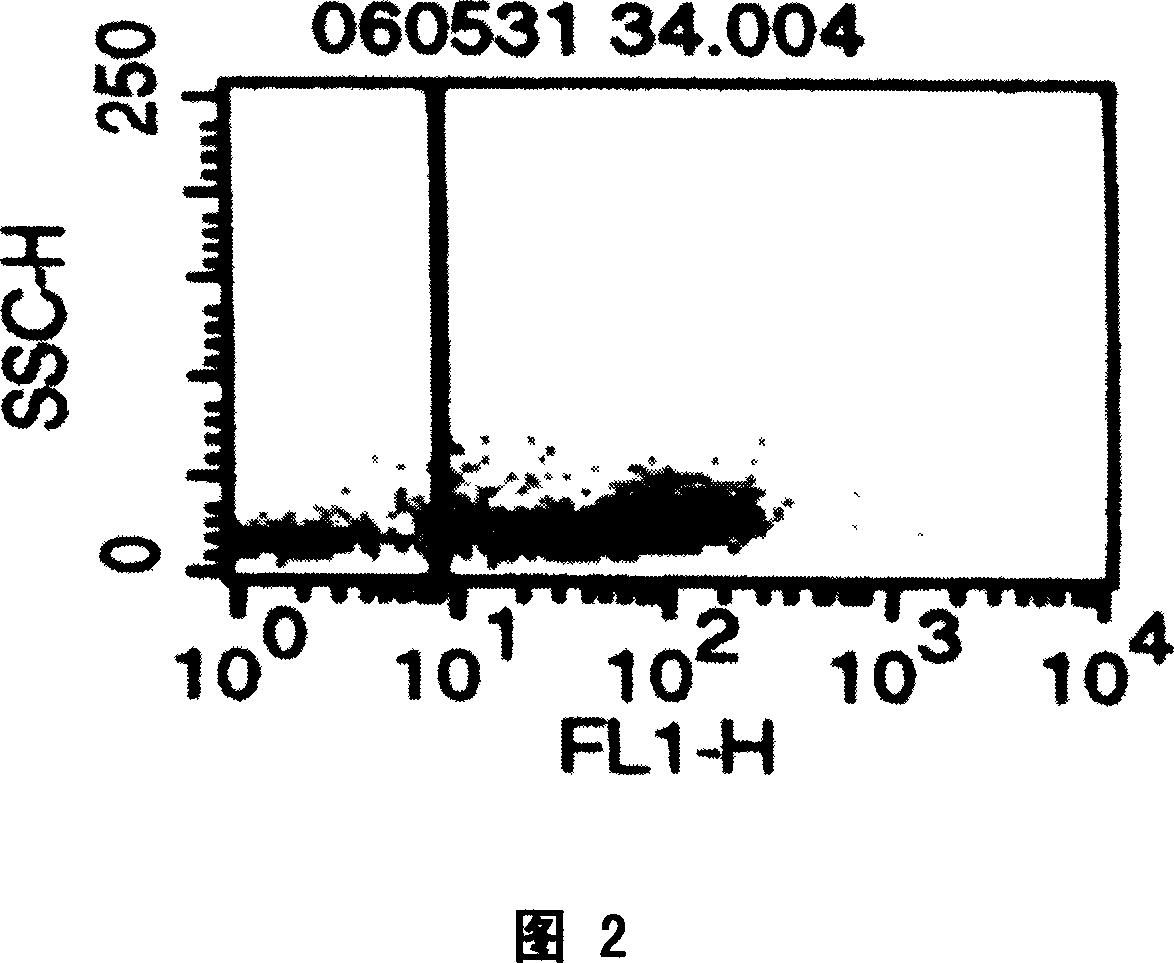
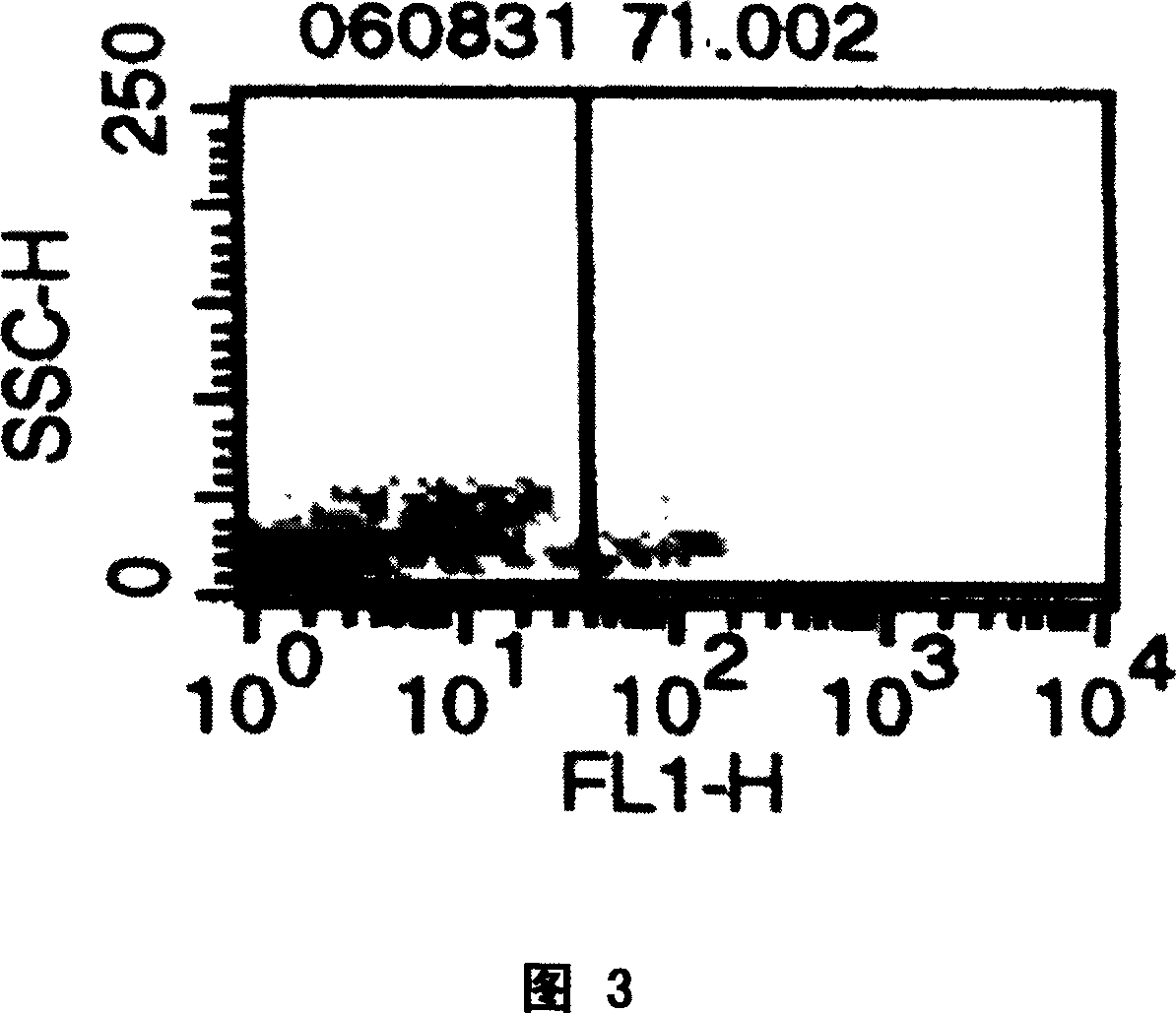
[0052] As shown in Figure 1, the donor-type human / pig hematopoietic chimera was constructed by injecting human-derived hematopoietic factors
[0053] Step 11: Preparation of hHSC for transplantation by separating, purifying and expanding human mononuclear cells (hMNC)
[0054] A) Using human umbilical cord blood mononuclear cells to separate and purify hHSC;
[0055] The hHSCs used are mainly derived from umbilical cord blood mononuclear cells. Healthy, full-term mothers are selected, and the umbilical cord blood is collected after the fetus is delivered naturally or after caesarean section, and sodium citrate is used for anticoagulation. Anticoagulated umbilical cord blood was thoroughly mixed with an equal amount of RPMI-1640 culture medium, and the lymphocyte separation medium was used to separate mononuclear cells (MNC) according to MACS-CD34 + Immunomagnetic Beads Separation Kit Instructions Carry out magnetic labeling on MNC, the labeled cells pass through a sorting col...
Embodiment 2
[0076] Construction of donor-type human-pig hematopoietic chimera by transfection of human gene
[0077] Similar to Example 1, the difference is:
[0078] 21) Immediately transplant human bone marrow mesenchymal stem cells transfected with human hematopoietic factors such as human erythropoietin (hEPO) after transplanting human hematopoietic stem cells (hHSC) to the selected suckling pigs.
[0079] Wherein, human bone marrow mesenchymal stem cells transfected with human hematopoietic factor genes are prepared through the following steps:
[0080] Step 211) Cloning human-derived hematopoietic factor genes such as hEPO. Retrieve the gene sequence from the gene bank (GenBank), design amplification primers (introduce restriction sites BamH I and Xho I), perform RT-PCR, recover the amplified fragment, and insert it into the eukaryotic expression vector (pcDNA3) after the sequence is correct. middle.
[0081] Among them, RT-PCR is an existing technology that combines reverse tran...
Embodiment 3
[0086] Construction of human-pig hematopoietic chimeras using transgenic pigs
[0087] Similar to Example 1, the difference is:
[0088] 31) The suckling pigs selected for the construction of human / pig hematopoietic chimeras are transgenic pigs.
[0089] 32) For the screened transgenic pigs, there is no need to inject human-derived hematopoietic factors, such as rhEPO, etc. after hHSC transplantation.
[0090] The application of the donor hematopoietic human / pig hematopoietic chimera of the present invention is further described in detail below:
[0091] A) Application of the donor-type human / pig hematopoietic chimera of the present invention as providing human red blood cells
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com