Improved inactivated FCV vaccines
A technology of immunogenicity and immune response, which is applied in the field of inducing immune response and producing FCV immunogenic compositions, and can solve problems such as vaccine safety problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Inactivation of FCV by formaldehyde
[0080]CRFK cells (Crandell-Reese cat kidney cells, available as CCL-94 from the American Type Culture Collection) were cultured in 2-liter roller bottles (850 cm 2 ), a modified Eagle medium (MEM, Gibco BRL) supplemented with 2.5% whey protein hydrolyzate and 5% fetal bovine serum was used. Each roller bottle was filled with 300 ml of cell suspension in MEM medium containing approximately 100,000 cells / ml. After 3 days, the cell layer became confluent. The cell culture medium was then replaced with serum-free MEM and diluted with 0.5 CCID 50 The multiplicity of infection (MOI) per cell was added to strain 431 FCV virus. Virus cultures were maintained at 37°C for 24 to 48 hours until cytopathic effects were obtained on the entire cell layer. The virus suspension was harvested, then clarified on filters with a porosity of 1.5 μm and stored at 5°C. FCV viral titer at harvest was 8.5+ / -0.3log 10 CCID 50 / ml.
[0081]...
Embodiment 2
[0086] Embodiment 2: the stabilizing effect of formaldehyde on FCV under various conditions
[0087] The FCV 431 strain was grown essentially as described in Example 1.
[0088] Three inactivation methods were tested:
[0089] 1) Aziridine at a concentration of about 4mM for 24 hours at 20°C,
[0090] 2) Aziridine at a concentration of about 8 mM for 24 hours at 20°C,
[0091] 3) Aziridine at a concentration of about 8 mM for 24 hours at 5°C.
[0092] The virus suspension was stabilized for 5 days at 5°C (+ / - 3°C) by various final concentrations of formaldehyde from 0.1 g / l to 0.5 g / l. The control suspension contained no formaldehyde.
[0093] FCV p66 protein was quantified by ELISA titration as described in Example 1 before and after selective PEG precipitation. ELISA titers expressed as log 10 OD 50 .
[0094] formaldehyde,
[0095] These results demonstrate the stabilizing effect of 0.1 g / l and 0.5 g / l formaldehyde solutions under various inactivation cond...
Embodiment 3
[0096] Example 3: Immunogenicity of inactivated FCV virions
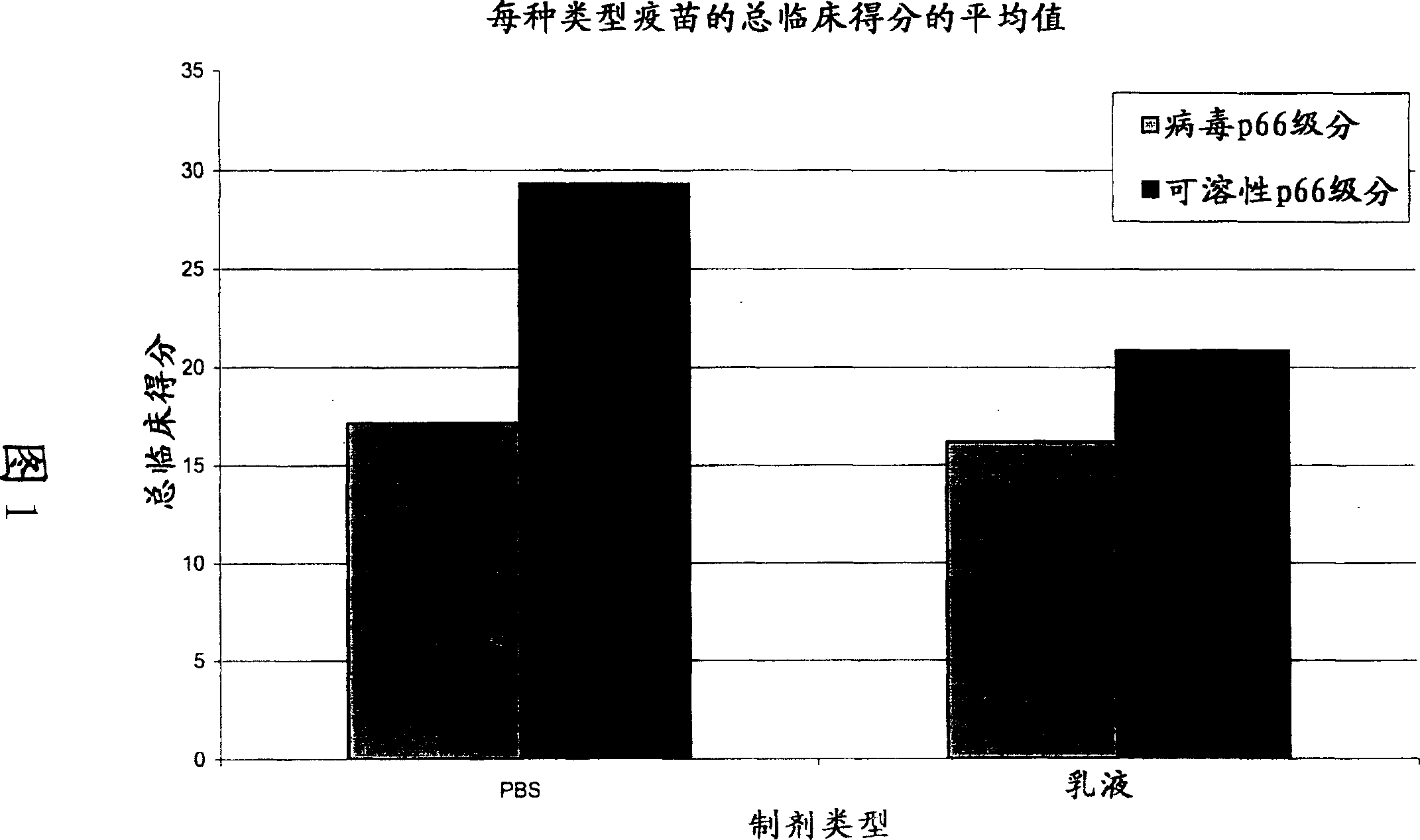
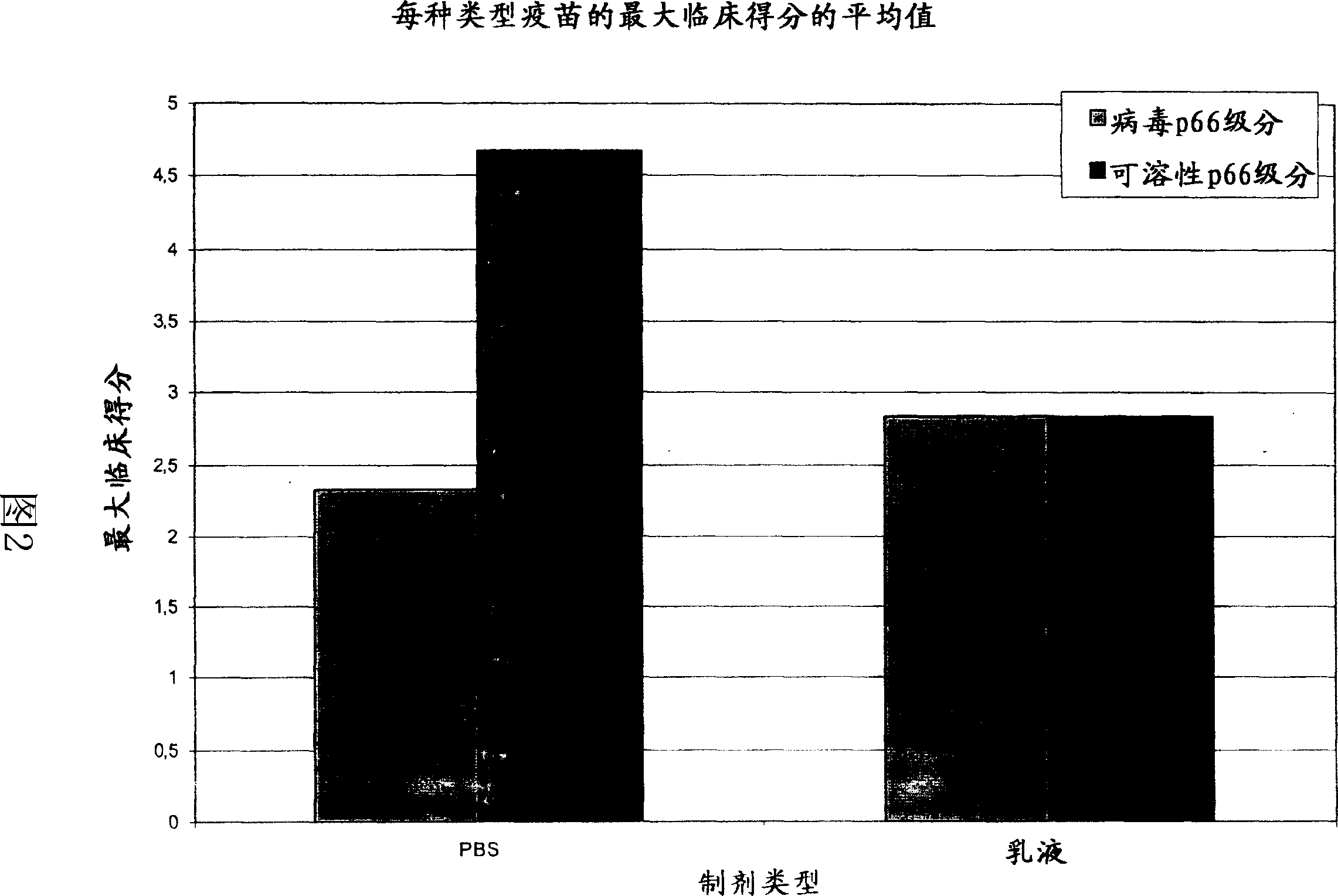
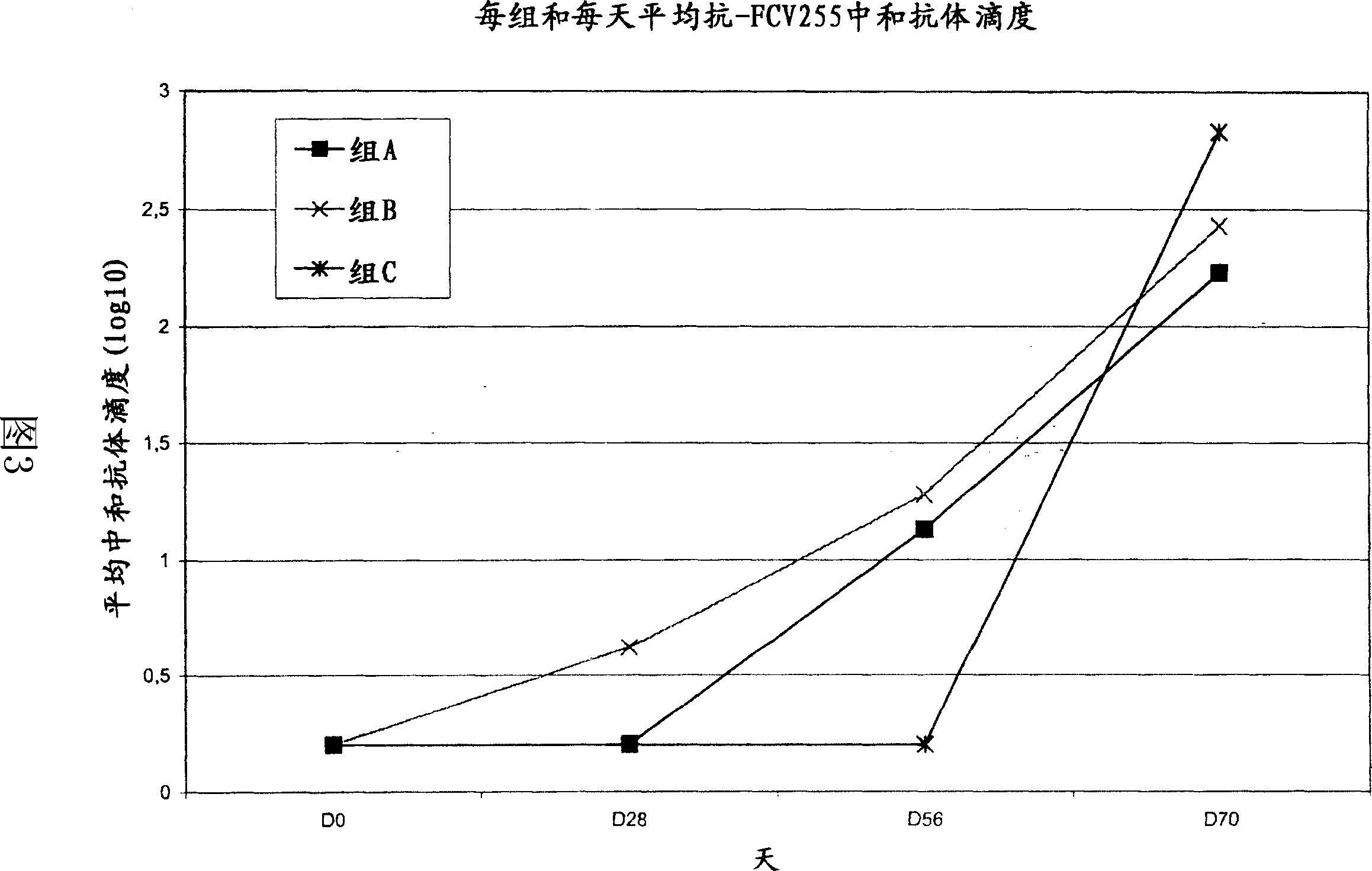
[0097] The FCV 431 strain was grown, inactivated and stabilized essentially as described in Example 1. The virus suspension was separated by size exclusion chromatography into two fractions containing virions (also called viral p66 fraction) and soluble p66 protein (also called soluble p66 fraction), respectively. After separation, the two fractions were stored at 5°C.
[0098] A total of 12 vaccines were prepared containing 1 ml of the virus p66 fraction diluted or undiluted (1 / 1, 1 / 4 and 1 / 16) and formulated with 1 ml PBS (phosphate buffered saline; no calcium and no magnesium); diluted or undiluted 1 ml viral p66 fraction diluted (1 / 1, 1 / 4 and 1 / 16) and formulated with 1 ml oil-in-water emulsion (paraffin oil, fatty alcohol ethers and polyols, polyoxyethylene fatty acids); diluted or undiluted (1 / 1, 1 / 4 and 1 / 16) and 1 ml soluble p66 fraction in PBS; diluted or undiluted (1 / 1, 1 / 4 and 1 / 16) and 1 ml soluble p6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com