Use of aerosolized antithrombin to treat acute lung injury
An acute lung injury and antithrombin technology, which is applied to medical preparations containing active ingredients, peptide/protein components, pharmaceutical formulas, etc., can solve the problems of no improvement in lung injury treatment and no inhibition of fibrin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
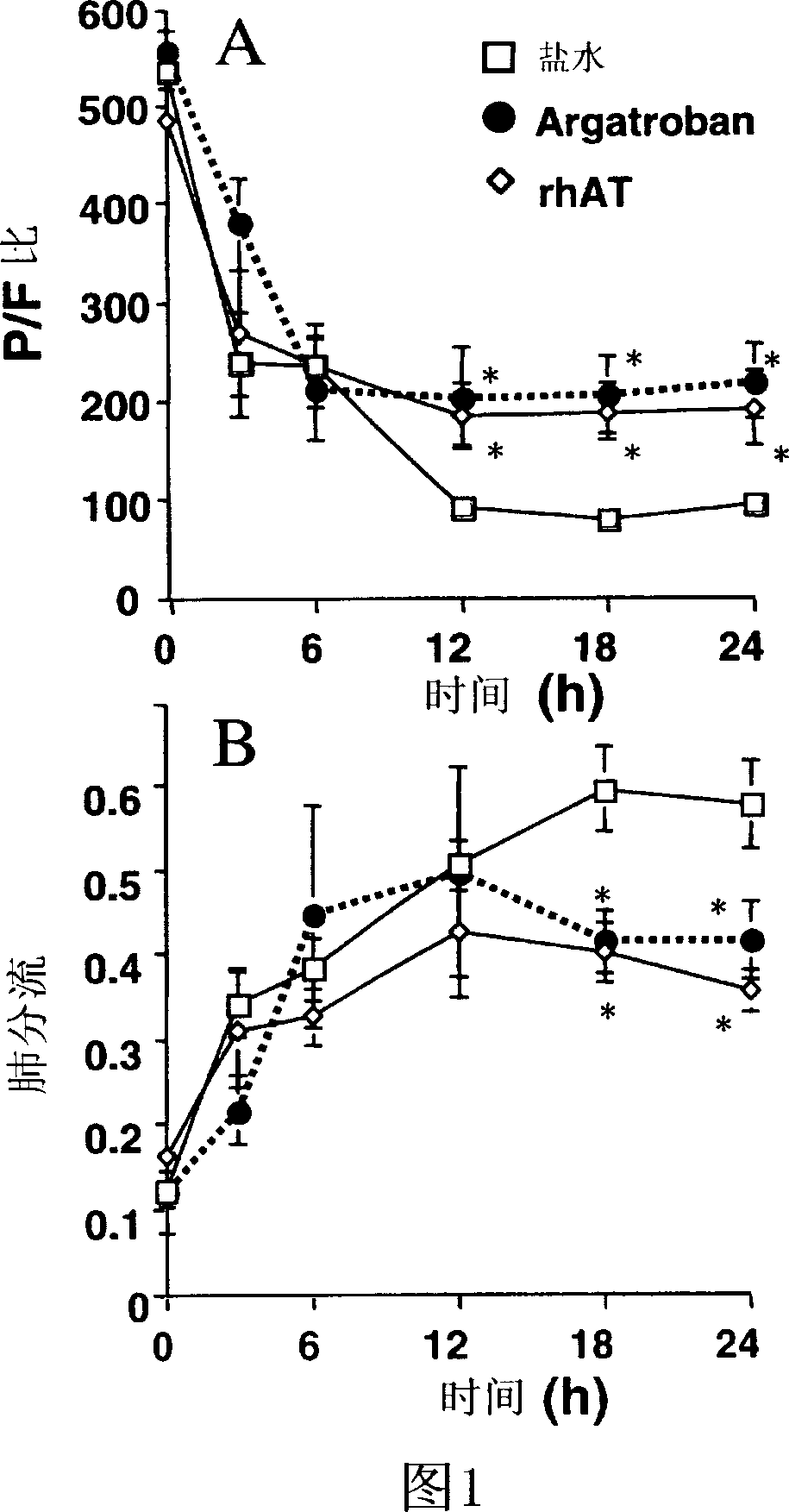
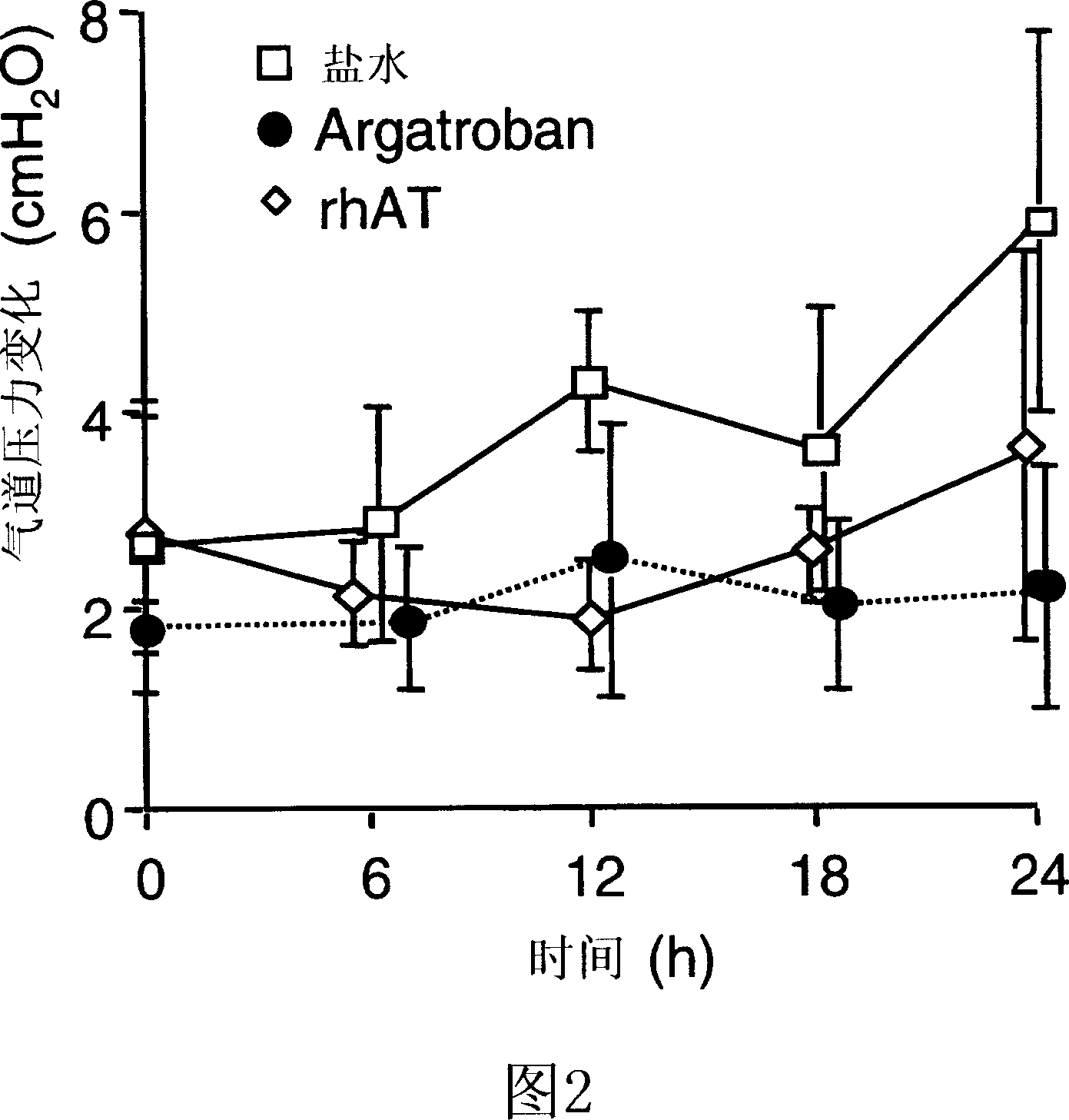
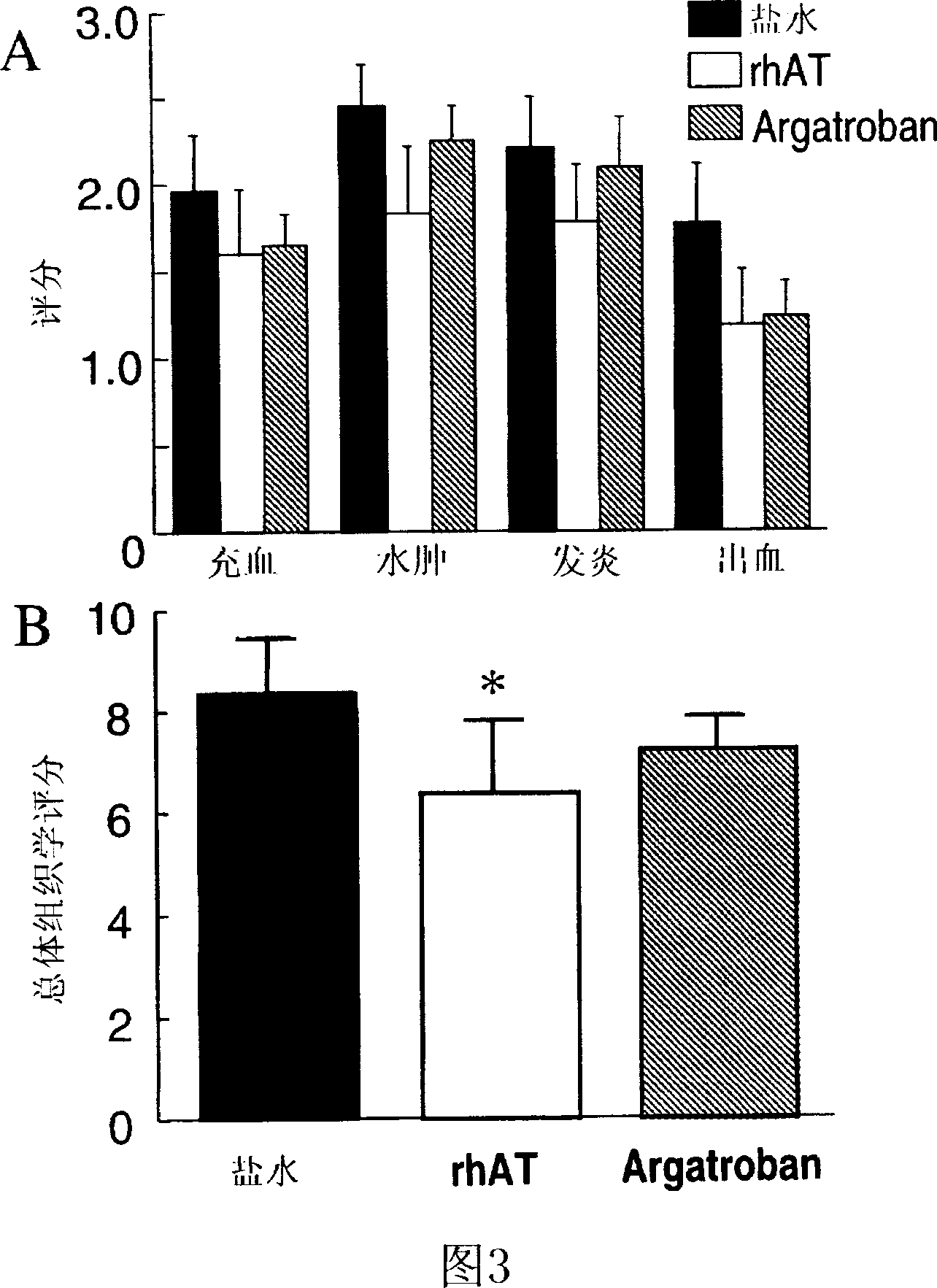
[0081] Recombinantly produced ATIII was dissolved in saline (42 mg / ml). Ewe sheep (n=24) were used. After 48 breaths of cotton smoke (11 ). All animals used 100% O 2 Mechanical ventilation (Tidal volume 15ml / kg, 30 breaths / min, PEEP 5cmH 2 O). At 2, 4, 8, 12, 16, and 20 hours after injury, recombinant antithrombin (n=6), argatroban (n=8), or the same volume of normal saline (n=10) were sprayed with an ultrasonic nebulizer. No animals died during the study. PaO 2 / FiO 2 Ratio, pulmonary shunt, and histological airway obstruction score were all reduced by antithrombin and argatroban. However, histopathological changes and lung wet / dry weight ratio were only reduced by antithrombin but not by argatroban.
[0082] Materials and methods
[0083] Material
[0084] Human recombinant antithrombin (ATIII) (7, 8) was a gift from GTC Biotherapeutics (Framingham, MA). Argatroban was purchased from Daiichi Pharmaceutical Co. (Tokyo, Japan). All other...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com