Application of beta-protein inhibitor in preparation of medicament for treating genetic correlated disease
A technology for inhibiting proteins and diseases, applied in genetic material components, drug combinations, gene therapy, etc., can solve problems such as the impact of acetylation levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0035] 1 Materials and methods
[0036] 1.1 Antibodies and Reagents
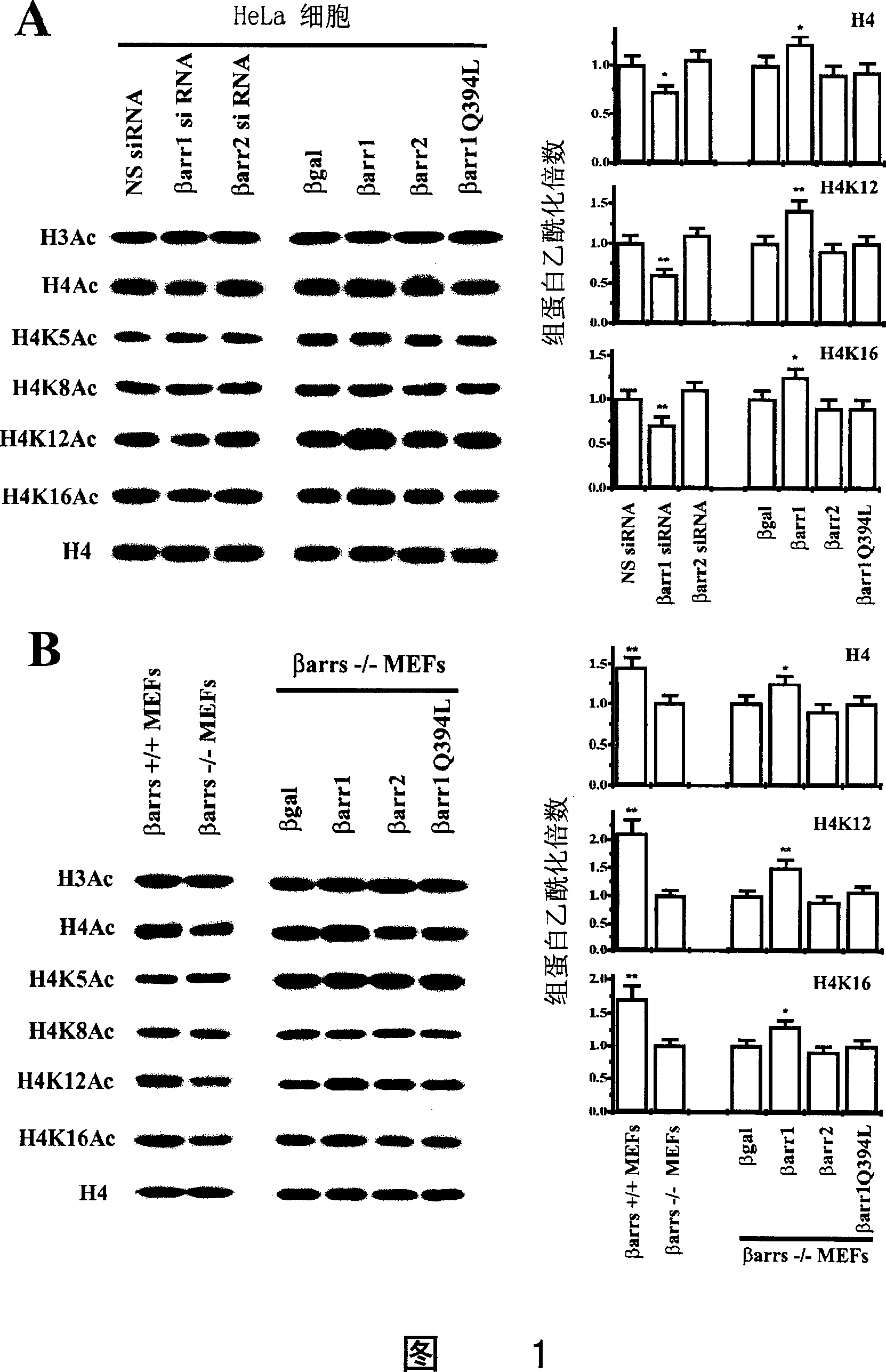
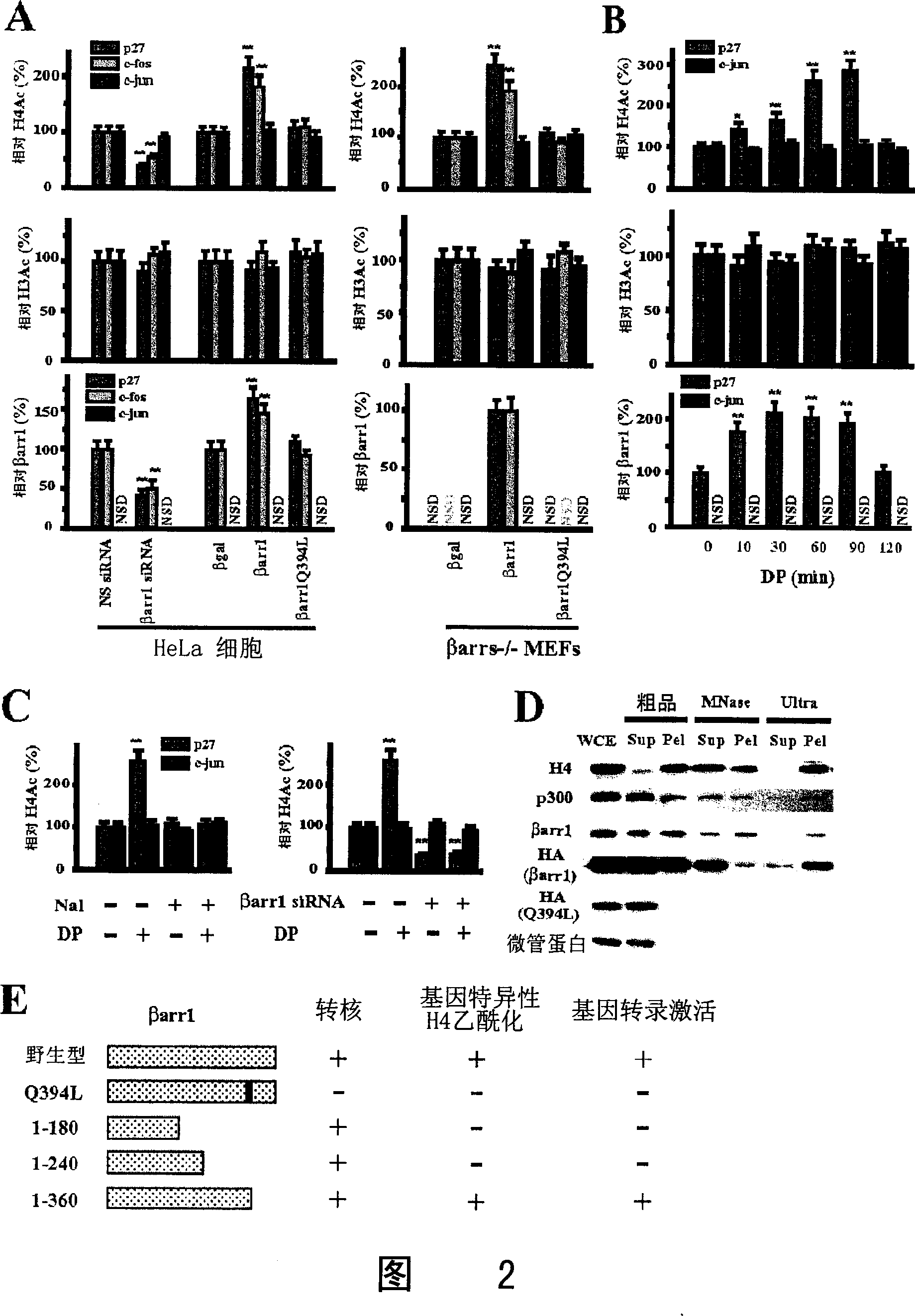
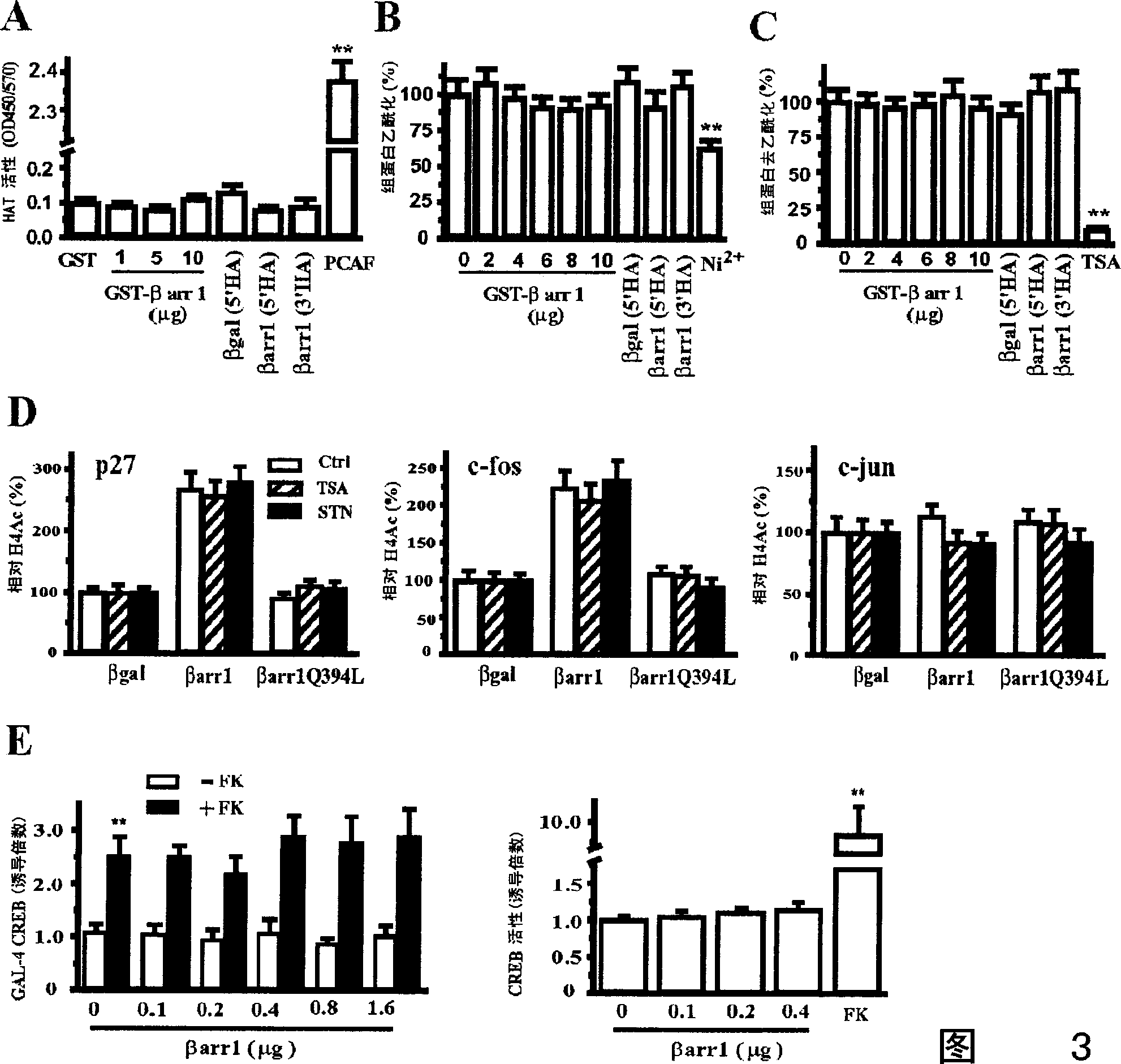
[0037]Beta-arrestin 1 mouse anti, p27 and c-Jun antibodies were purchased from BD Biosciences (San Jose, CA). Beta-arrestin polyclonal rabbit antibody was a gift from Robert J. Lefkowitz (Duke University Medical Center, Durham). p300 CT, CBP, histone H4, acetylated histone H4 and H3 antibodies were purchased from Upstate Biotechnology (Lake Placid, NY). Mouse anti-12CA5 against HA-tag was purchased from Roche Molecular Biochemicals (Indianapolis, IN). Tip60 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). CREB antibody was purchased from Cell Signaling Technology (Beverly, MA). Texas Red-conjugated goat anti-mouse IgG and FITC-conjugated goat anti-rabbit IgG were purchased from Jackson Immunoresearch (West Grove, PA). IRDye TM 800CW-conjugated affinity purified anti-mouse IgG and anti-rabbit IgG were purchased from Rockland Inc. (Gilbertsville, PA). The following antibodies were ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com