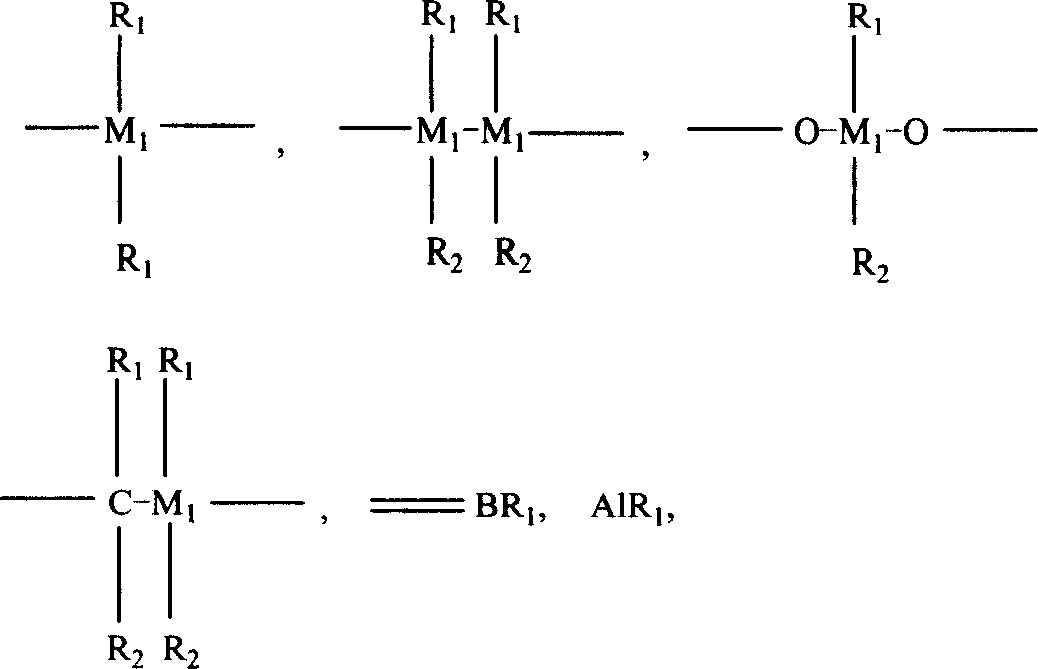
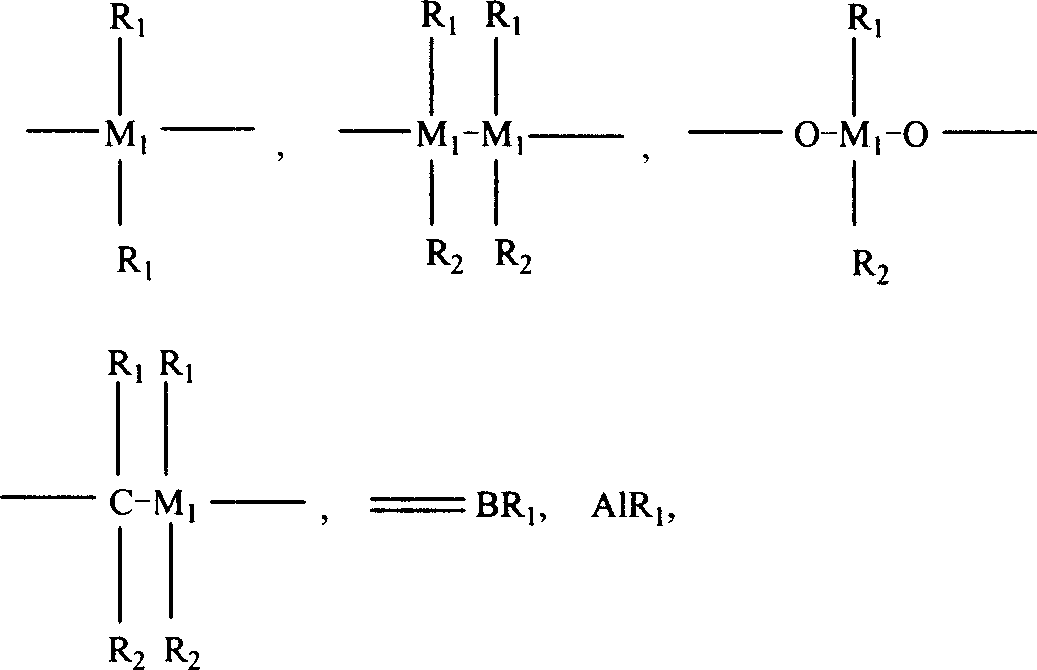
Composite catalyst, its production and use in polyolefine alloy
A composite catalyst and catalyst technology, applied in the field of composite catalyst and its preparation, can solve problems such as difficulty in uniform distribution of metallocene components, difficulty in sticking to a kettle or material transfer, and difficulty in industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Preparation of Composite Catalyst A
[0057] Under nitrogen protection, add 100ml TiCl to a 500ml reaction bottle with sand core filter and mechanical stirring at the bottom 4 , cooled to -20°C, and added 5 g spherical carrier MgCl 2 • nEtOH, reacted for 1 hour. Raise the temperature to 50°C, add 0.7g of diisobutyl phthalate, slowly raise the temperature to 120°C, react for 2 hours and filter, then add 100ml of TiCl 4 , reacted at 120°C for 2 hours. The product was washed with hexane at 60°C to obtain component a.
[0058] 0.03g C 2 h 4 (Ind) 2 ZrCl 2 After reacting with 0.02mol methylaluminoxane (MAO) at 20°C for 2 hours, add it to a, and react at 20°C for 2 hours. The obtained product was thoroughly washed with hexane, and dried under vacuum at room temperature for 1 hour. The composition of the obtained composite catalyst A is: 3.3 wt% of Ti, 0.17 wt% of Zr, 6.3 wt% of Al, 12.4 wt% of Mg, and 10.2 wt% of diisobutyl phthalate.
Embodiment 2
[0060] Preparation of Composite Catalyst B
[0061] Under nitrogen protection, add 100ml TiCl to a 500ml reaction bottle with sand core filter and mechanical stirring at the bottom 4 , cooled to -20°C, and added 5 g spherical carrier MgCl 2 • nEtOH, reacted for 1 hour. Raise the temperature to 60°C, add 0.65g of 9,9-bis(methoxymethyl)fluorene (BMF), slowly raise the temperature to 120°C, react for 2 hours and filter, then add 100ml of TiCl 4 , reacted at 120°C for 2 hours. The product was washed with hexane at 60°C to obtain component b.
[0062] 0.1g C 2 h 4 (Ind) 2 ZrCl 2 After reacting with 0.05mol methylaluminoxane (MAO) at 20°C for 2 hours, add it to b, and react at 20°C for 2 hours. The obtained product was thoroughly washed with hexane, and dried under vacuum at room temperature for 1 hour. The composition of the obtained composite catalyst B is: Ti 2.8wt%, Zr 0.83wt%, Al 9.8wt%, Mg 9.8wt%, BMF 9.5wt%.
Embodiment 3
[0064] Preparation of Composite Catalyst C
[0065] Under nitrogen protection, add 100ml TiCl to a 500ml reaction bottle with sand core filter and mechanical stirring at the bottom4 , cooled to -20°C, and added 5 g spherical carrier MgCl 2 • nEtOH, reacted for 1 hour. Raise the temperature to 60°C, add 0.65g of 9,9-bis(methoxymethyl)fluorene (BMF), slowly raise the temperature to 120°C, react for 2 hours and filter, then add 100ml of TiCl 4 , reacted at 120°C for 2 hours. The product was washed with hexane at 60°C to obtain component c.
[0066] 0.7g Me 2 Si(1-Me-7-benzoindenyl) 2 ZrCl 2 After reacting with 0.03mol methylaluminoxane (MAO) at 20°C for 2 hours, add it to c, and react at 20°C for 2 hours. The obtained product was thoroughly washed with hexane, and dried under vacuum at room temperature for 1 hour. The composition of the obtained composite catalyst C is as follows: Ti 2.9wt%, Zr 0.45wt%, Al 8.1wt%, Mg 11.3wt%, BMF 11.4wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com