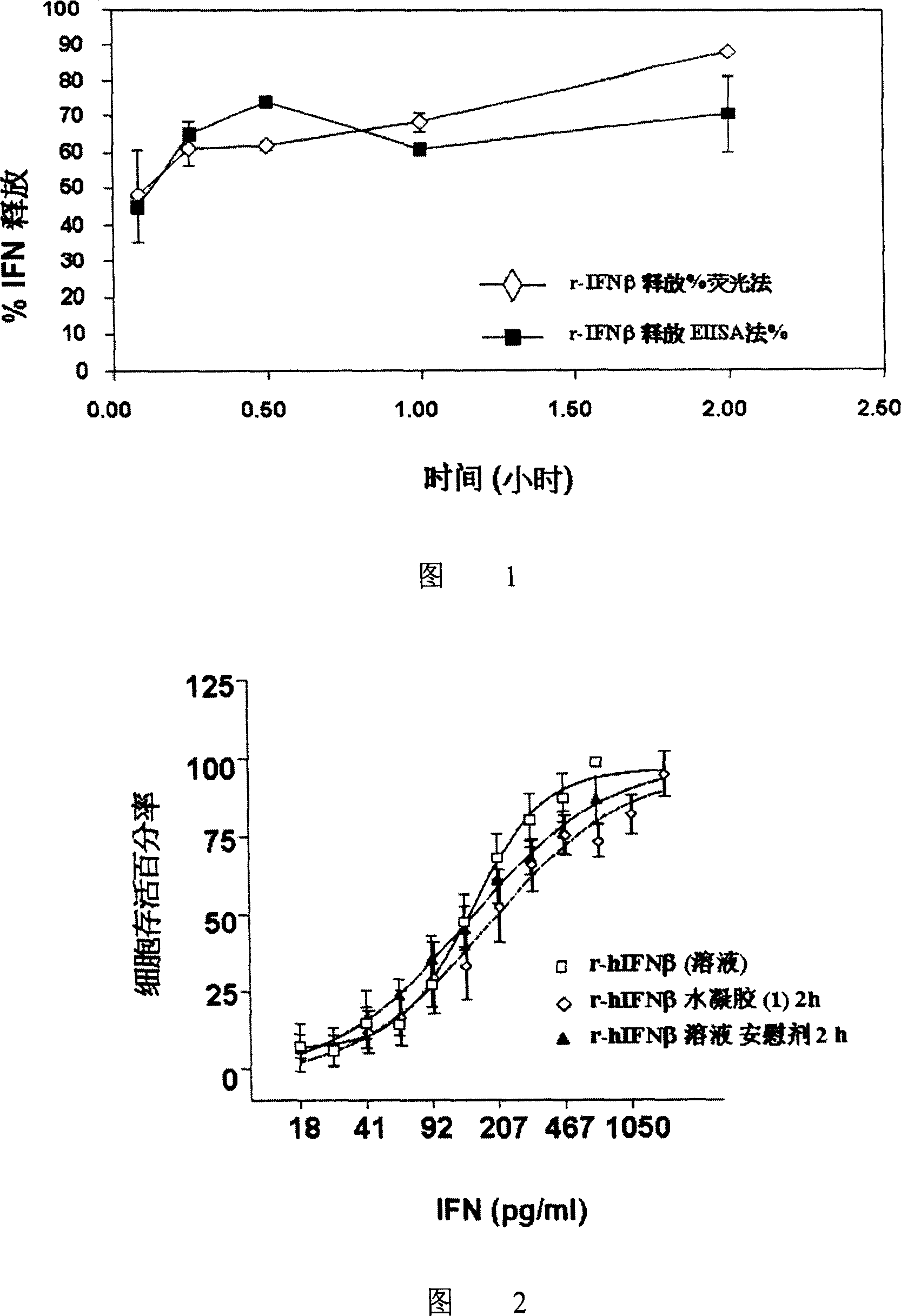
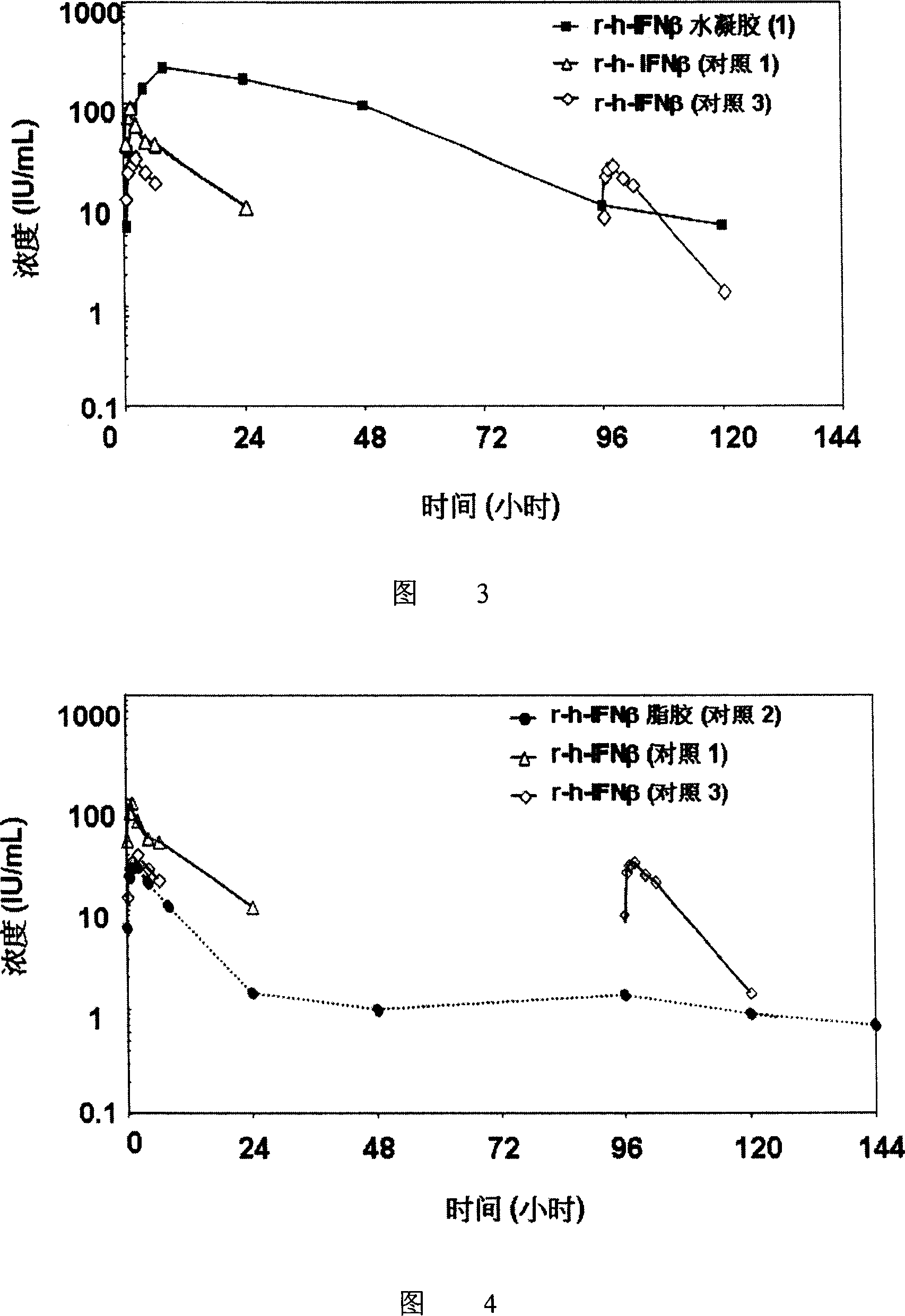
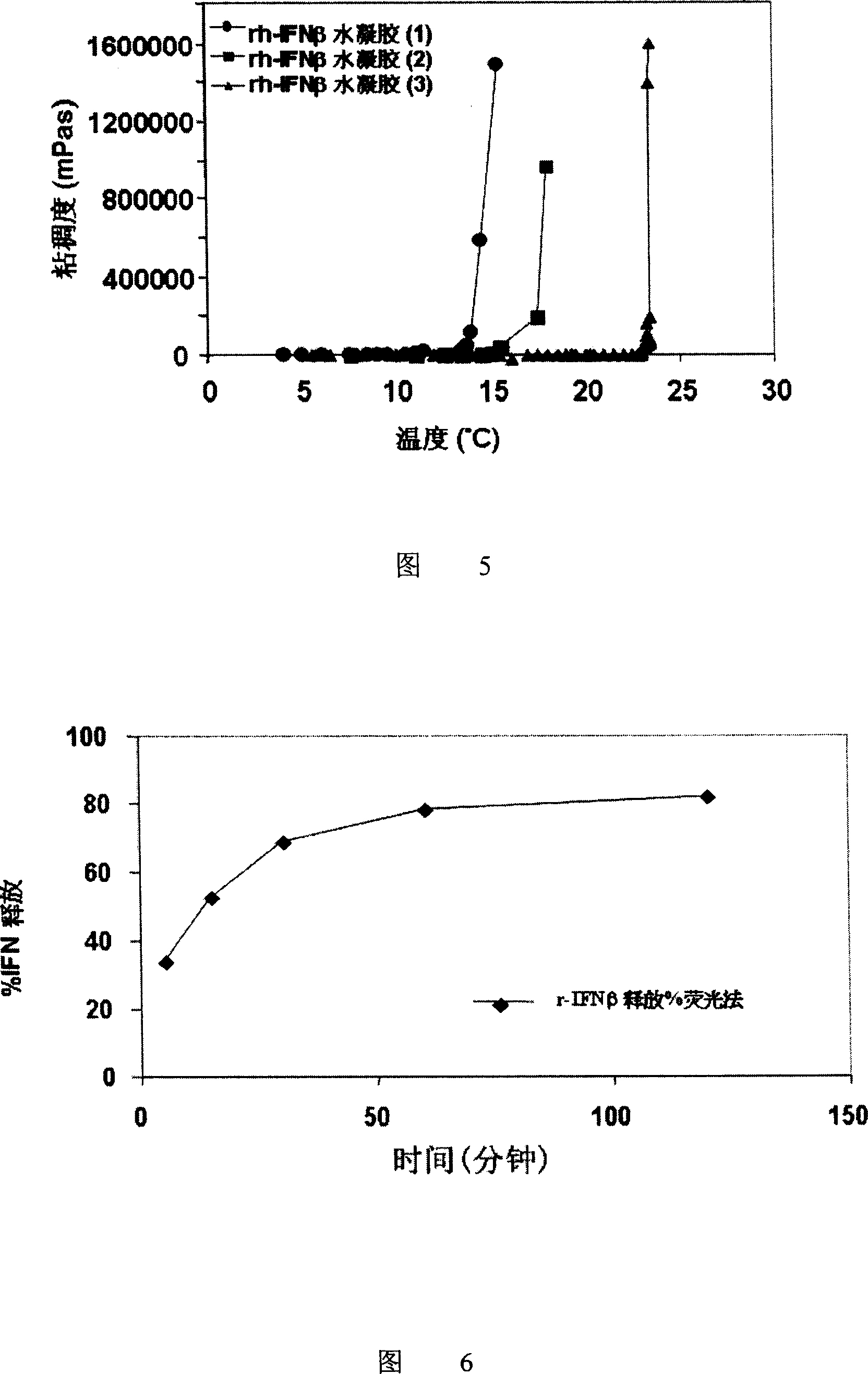
Hydrogel interferon formulations
A technology of interferon and hydrogel, applied in the direction of non-active ingredient medical preparations, active ingredient-containing medical preparations, cyclic peptide ingredients, etc., to achieve the effect of easy operation and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] Unless the broader meaning of this definition is specifically stated otherwise, the following paragraphs will define the different chemical components that constitute the compound of the present invention and apply to the entire specification and claims.
[0037] "Interferon" or "IFN" as used herein means any molecule as defined in the literature, including, for example, any type of interferon mentioned in the background of the invention. Specifically, IFN-α, IFN-β, and IFN-γ are included in the above definition. The interferon of the present invention is preferably IFN-β. IFN-β suitable for the present invention can be obtained through commercial products, such as Rebif (Serono), Avonex (Biogen) or Betaferon (Schering).
[0038] As used herein, the term "interferon-β (IFN-β)" refers to include: fibroblast interferon, especially human fibroblast interferon, refers to isolated and obtained from biological fluids or obtained from eukaryotic or prokaryotic cells by recom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| transition temperature | aaaaa | aaaaa |
| water solubility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com