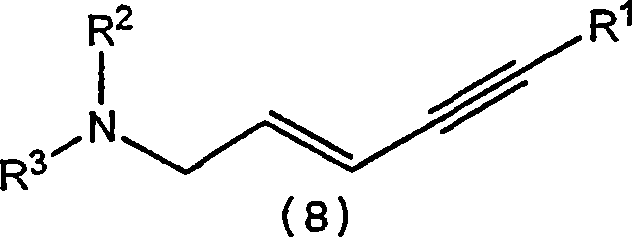
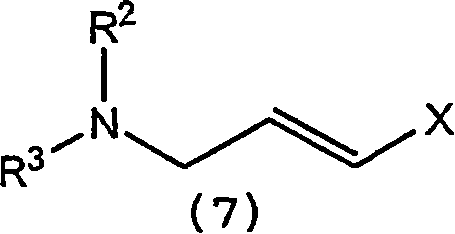
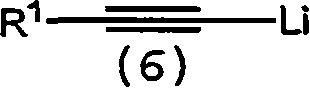A process for the synthesis of terbinafine and derivatives thereof
A compound, straight-chain technology, applied in the field of synthesis of terbinafine and its derivatives, can solve the problems of final drug price impact, unsuitability for industrial production, social consequences, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1: 1,1-dichloro-3,3-dimethylbutene
[0090] Add FeCl to a reactor filled with inert gas 3 50.0 g (0.3 mol) and 350 ml of dichloromethane. At a temperature of 20-25°C, a mixture containing 478ml (407g; 4.39mol) of tri-butyl chloride and 380ml (426g; 4.39mol) of vinylidene chloride was added dropwise to the aforementioned suspension, and three Hour. The half-quantity method is to add 20g (0.123mol) FeCl 3 solution, and finally an additional 5 g (0.07 mol) was added. After the reaction mixture was stirred for 2 hours, the suspension was poured into a reactor containing 500 ml of water. The two phases were separated and the organic phase was washed with 250 ml of water followed by 250 ml of an aqueous solution of NaHCO3 (5% w / w).
[0091] The organic phase was distilled under vacuum conditions to obtain 395g (2.58mol-58% yield) of 1,1-dichloro-3,3-dimethylbutene (the component peak of the fraction was P=140mbar; T=65°C ), the purity is 95.1% GC (A%)-(the perce...
Embodiment 2
[0092] Example 2: 1,1-dichloro-3,3-dimethylbutene
[0093] Add FeCl to a reactor filled with inert gas 3 50.0 g (0.3 mol) and 350 ml of 1,2,4-trichlorobenzene. At a temperature of 20-25°C, a solution containing 478ml (407g; 4.39mol) of tri-butyl chloride and 380ml (426g; 4.39mol) of vinylidene chloride was added dropwise to the aforementioned suspension, and 4 After stirring the reaction mixture for 16 hours, the suspension was poured into a reactor containing 500 ml of water. The two phases were separated and the organic phase was washed with 250 ml of water followed by 250 ml of an aqueous solution of NaHCO3 (5% w / w).
[0094] The organic phase was distilled to obtain 387 g (2.53 mol-57.6% yield) of 1,1-dichloro-3,3-dimethylbutene (composition peak value of the fraction was P=100 mbar; T=65° C.), purity It was 93.0% GC (A%).
Embodiment 3
[0095] Example 3: 1,1-dichloro-3,3-dimethylbutene
[0096] Add FeCl to a reactor filled with inert gas 3 25.0 g (0.15 mol) and 175 ml of nitrobenzene.
[0097] At a temperature of 20-25°C, a mixture containing 239ml (204g; 2.2mol) of tri-butyl chloride and 190ml (213g; 2.2mol) of vinylidene chloride was added dropwise to the aforementioned solution for 2 hours , and the reaction mixture was stirred for 2 hours.
[0098] The mixture was distilled under vacuum to give 150 g (0.98 mol-41% yield) of 1,1-dichloro-3,3-dimethylbutene (composition peak of the fraction at P=140 mbar; T=65° C.) , with a purity of 94.4% GC (A%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com