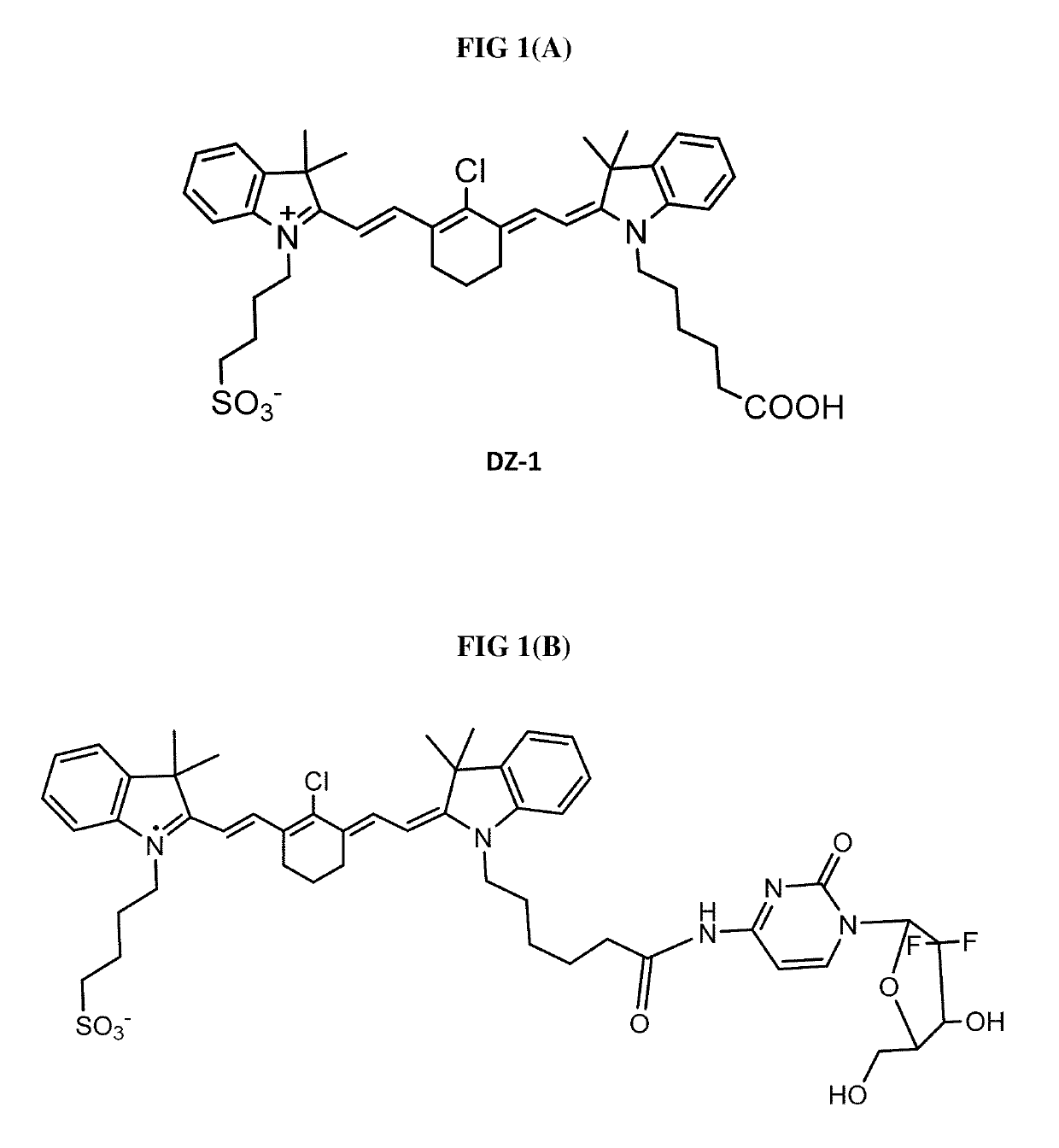
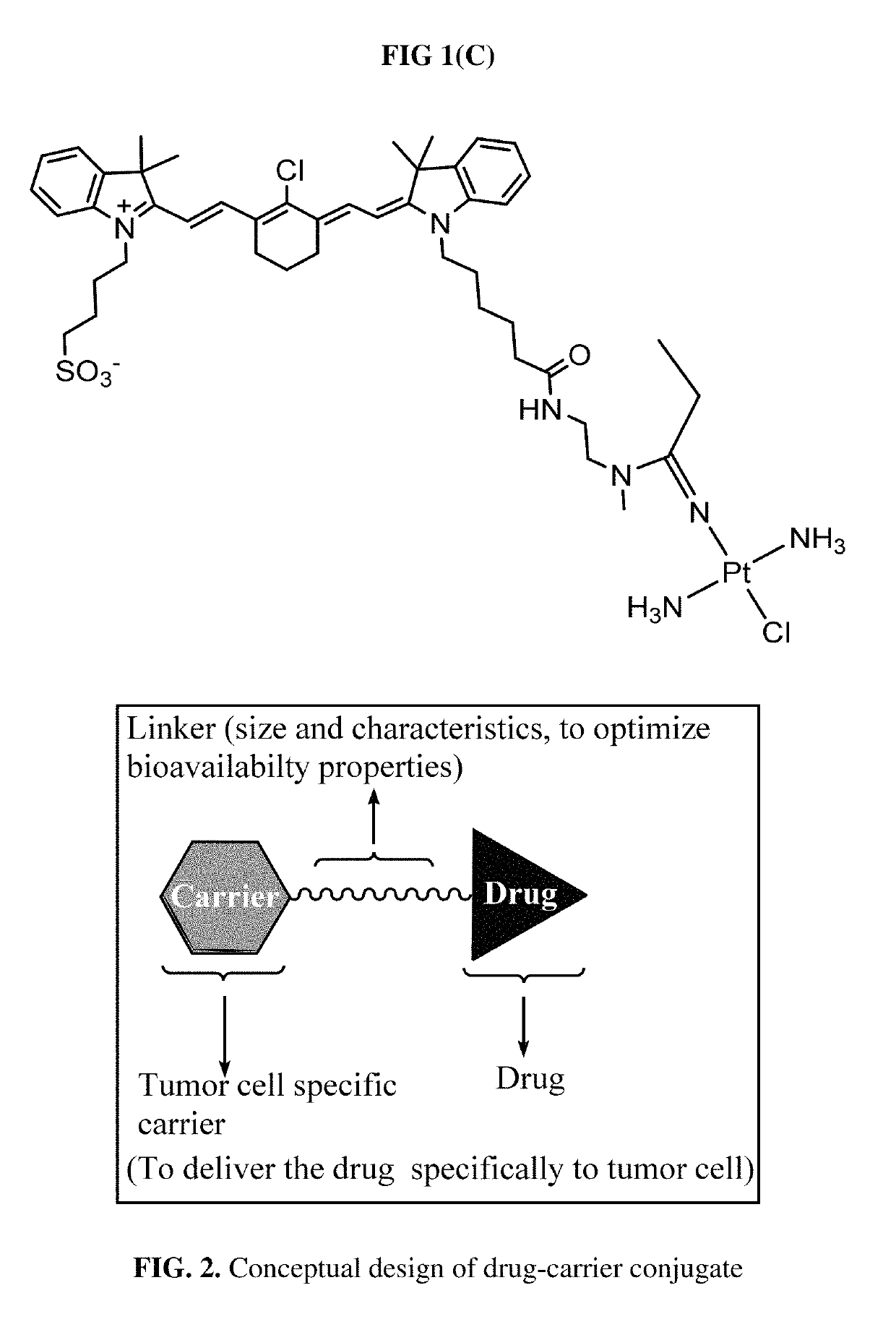
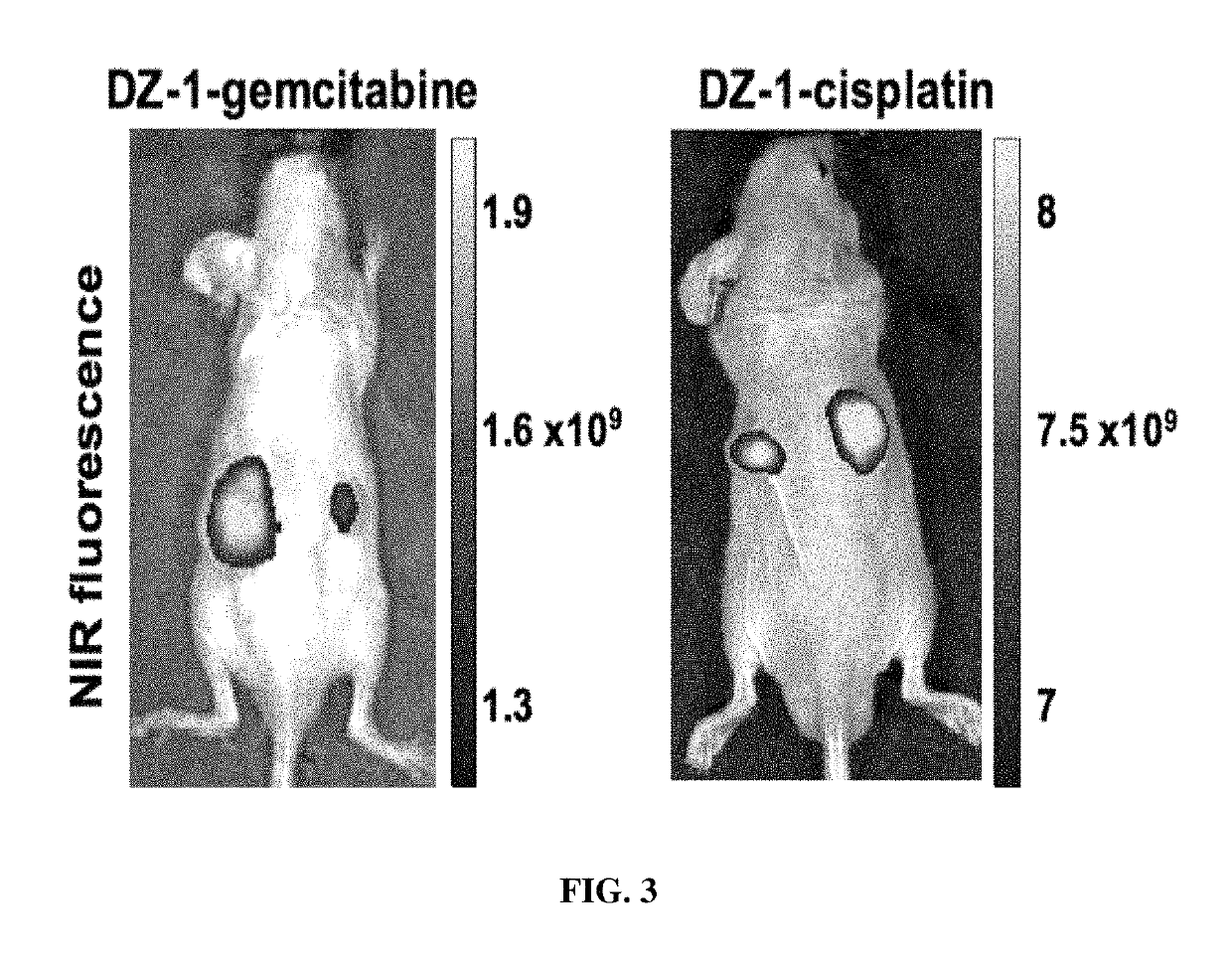
Organic anion transporting peptide-based cancer imaging and therapy
a technology of organs and anion transporters, applied in the field of organic anion transporting peptide-based cancer imaging and therapy, can solve the problems of no effectiveness therapy, achieve the effect of improving the efficacy of chemotherapy, reducing the number of side effects, and improving the uptake and retention of nir dye-drug conjuga
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0073]IR-783-gemcitabine (NIRG) Synthesis:
[0074]Synthesis of compound 5: To the mixture of 1a (2 g, 6.78 mmol) and Vilsmeier-Haack reagent 4 (3 g, 8.36 mmol) in EtOH (50 ml) was added CH3COONa (0.56 g, 6.78 mmol). The resulting mixture was heated under reflux for 3 h. The reaction mixture was concentrated, and the residue was recrystallized from methanol-ether to afford the desired product 5 as a dark blue solid (2 g, yield 56%). 1H NMR (DMSO-d6, 400 MHz) δ 10.20 (s, 1H), 8.43 (d, 1H, J=16 Hz), 8.19 (s, 1H), 7.71 (m, 2H), 7.53-7.39 (m, 6H), 7.16 (m, 1H), 6.62 (d, 1H, J=12 Hz), 4.38 (m, 2H), 2.71 (m, 4H), 2.52 (m, 2H), 1.87 (m, 4H), 1.75 (m, 2H), 1.69 (s, 6H). MS (ESI-TOF) [M+H]+: 525.1979.
[0075]Synthesis of heptamethine cyanine dye 6: To a mixture of 3b (0.5 g, 1.4 mmol) and compound 5 (1 g, 1.9 mmol) in EtOH (20 ml) was added CH3COONa (128 mg, 1.5 mmol). The resulting solution was heated under reflux for 3 h. The reaction mixture was concentrated, and the residue was purified with ...
example 2
[0078]The cisplatin nitrile complex 8:
[0079]A mixture of cisplatin 7 (0.6 g, 2.0 mmol) and propionitrile (8.2 mL, 118.2 mmol) in 30 mL of HCl (pH 4) was heated under reflux for 2 h. The solvent was removed under reduced pressure, and the pale-yellow residue was dissolved in 15 mL of methanol. The solution was filtered and the filtrate was added to 200 mL of ether. The precipitates were collected and dried under vacuum to afford 373 mg 8 (yield: 57%). MS (ESI-TOF) [M+H]+: 320.0271.
[0080]Synthesis of IR-783-N1-methylethylenediamine 9:
[0081]A mixture of 6 (850 mg, 1.2 mmol), 1-ethyl-3-(3-dimethyllaminopropyl) carbodiie hydrochloride (346 mg, 1.8 mmol), and 1-hydroxy-7-azabenzotriazole (195 mg, 1.4 mmol) were dissolved in 10.0 mL DMF. The mixture was stirred for 30 min, and then N-BOC-N-methylethylenediamin (231 mg, 1.7 mmol) was added. The mixture was stirred for additional 18 hours. Ethyl ether (50 ml) was added. The precipitates were collected and purified by C18-RP silica chromatogr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| time | aaaaa | aaaaa |
| wavelengths | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com