Fibronectin type III domain-based fusion proteins
a fibronectin type and domain technology, applied in the field of antibody mimetics, can solve the problem of critical delivery problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Design of Multivalent Protein-ELP Fusions
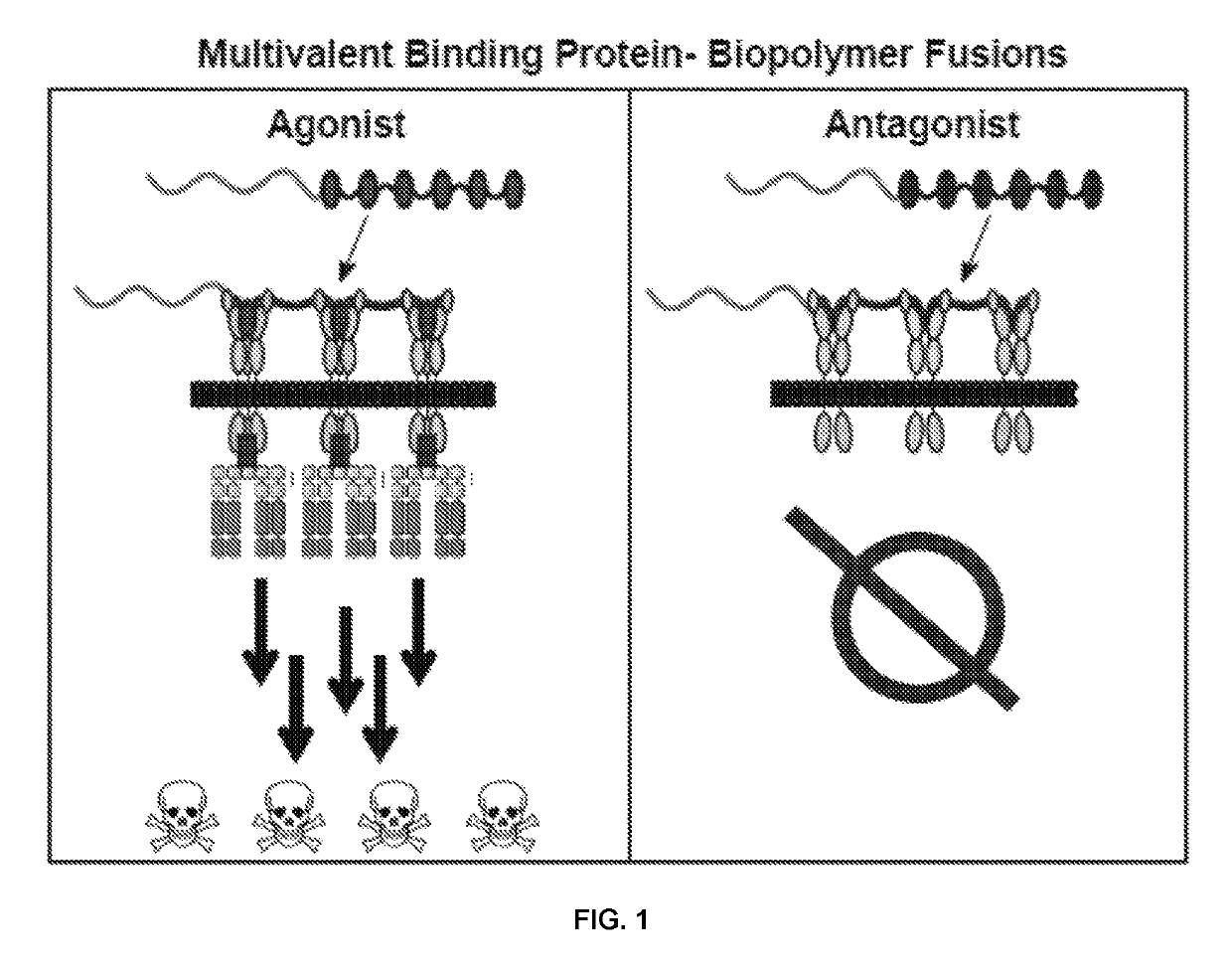
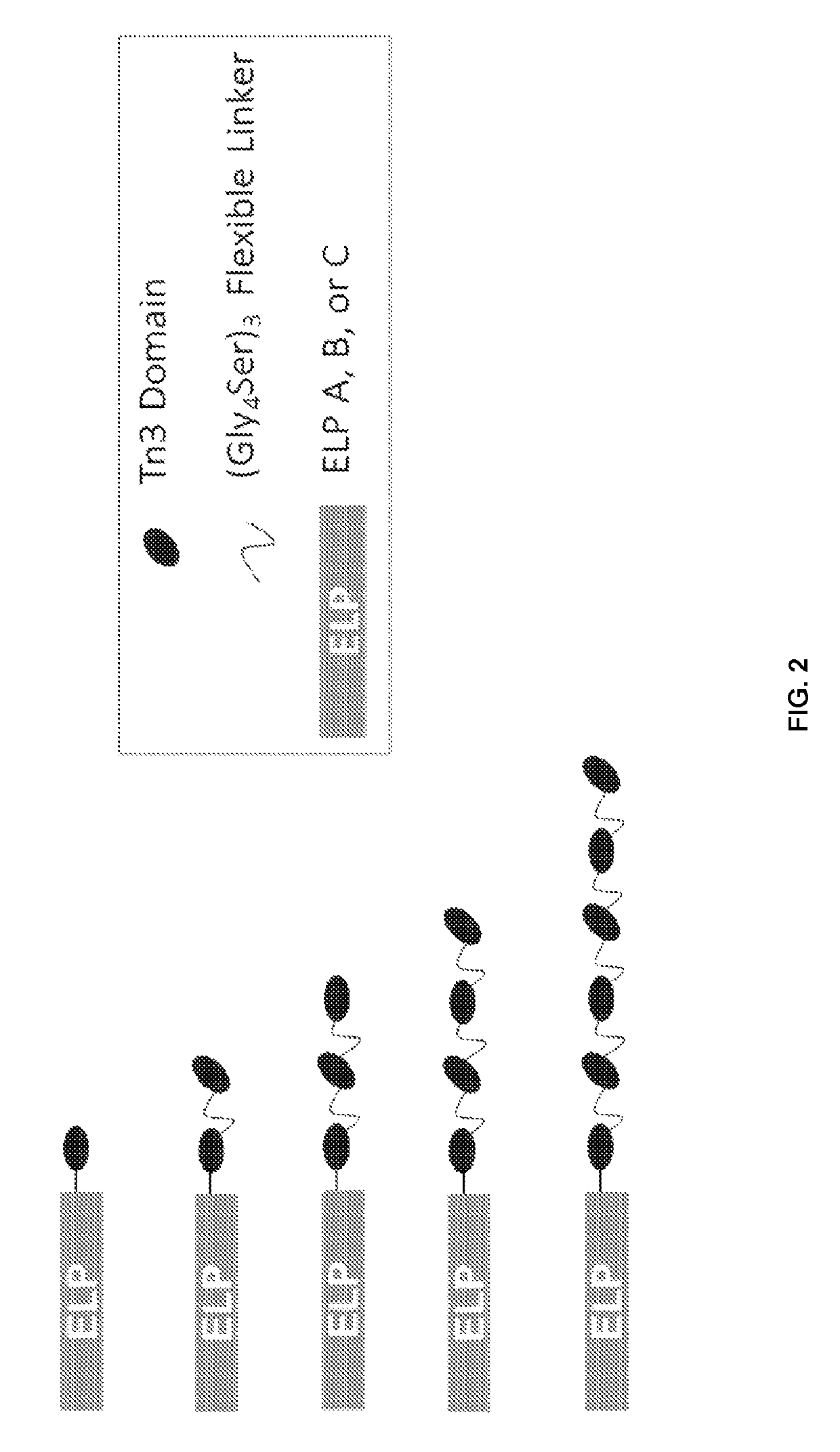
[0103]The fusion proteins included two parts (FIG. 1): (i) a multivalent targeting component (e.g., TRAILR-2 agonist or EGFR antagonist) protein in which one or more scaffold protein units (e.g., SEQ ID NO: 1 and 2 or 5) are linked by glycine-serine flexible (e.g., SEQ ID NO: 3) or structured proline-containing linkers (e.g., SEQ ID NO: 4); and (ii) an elastin-like-polypeptide connected to the multivalent protein (e.g., SEQ ID NO: 7-9).
[0104]The fusion of (i) to (ii) was at the N- or C-terminus or (ii) was interspersed among (i).
example 2
Design and Preparation of Multivalent Protein-ELP Expression Constructs
[0105]The DNA encoding the TRAILR-2-specific Tn3 unit (SEQ ID NO: 13; Swers et al., Mol. Cancer Ther., 2013, 12, 1235-1244) and the EGFR-specific domain (SEQ ID NO: 14; Friedman, et al., J. Mol. Biol. 2008, 376, 1388-1402) were purchased as double-stranded DNA “G-blocks” from Integrated DNA Technologies (Coralville, Iowa). The Tn3 G-block (SEQ ID NO: 13) was amplified using primers “Tn3For” and “Tn3Rev” primers (SEQ ID NO: 15 and 16, respectively). The gene was purchased with a (Gly4Ser)3 linker (SEQ ID NO: 3) at the C-terminus and designed with restriction sites compatible with recursive directional (RDL) ligation for seamless cloning of oligomeric genes. The EGFR-binding G-block (SEQ ID NO: 5) was purchased such that it could be inserted into the vector (SEQ ID NO: 12) using Gibson Assembly. The G-block contained 40-50 nucleic acid bases identical to those in the vector.
[0106]Enzymes used were from New England ...
example 3
Expression and Purification of Multivalent TRAILR-2 Agonist-ELP Fusion Proteins
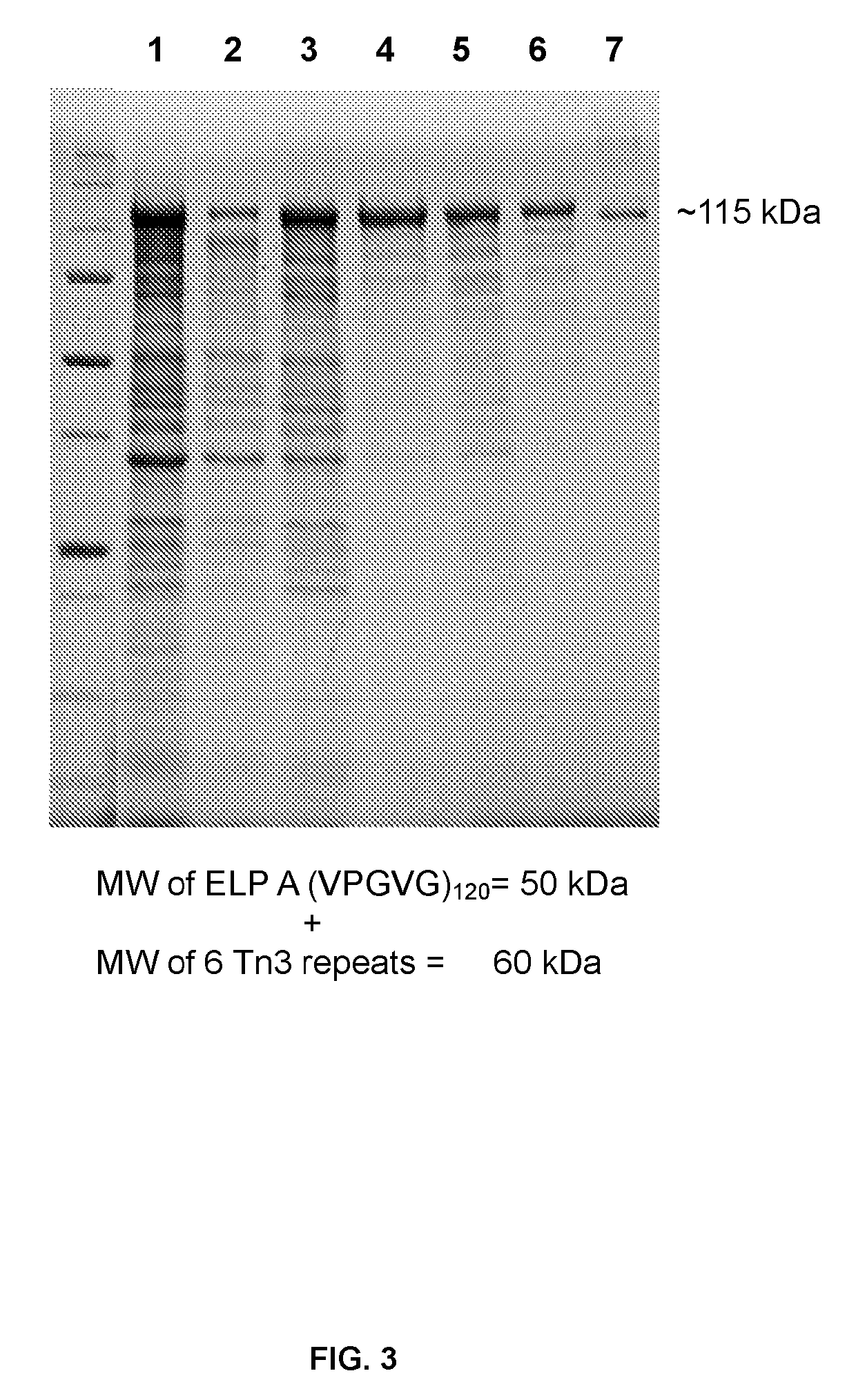
[0108]The multivalent ELP-(Tn3)6 fusion constructs (SEQ ID NO: 10 and 11; FIG. 2) were transformed into BL21(DE3) cells (EMD / Novagen, Gibbstown, N.J.) for expression. Transformants were grown in Terrific Broth (TB) containing 45 μg / mL kanamycin and incubated overnight at 37° C. with shaking. Overnight cultures were diluted 1 to 40 into TB containing 45 μg / mL kanamycin and incubated at 37° C. with shaking for 5-8 hours. Protein expression was then induced by addition of IPTG to 1 mM, and incubation was resumed at 37° C. with shaking. In a specific embodiment, the Tn3-ELP fusion proteins were purified from the cell lysate using inverse transition cycling (ITC) as previously described (Christensen et al., Protein Science 2009, 18, 1377-1387; Hassouneh et al., Methods Enzymol. 2012, 502, 215-37). In another embodiments, C-terminally His8-tagged ELP-Tn3 fusion proteins were purified from the periplasmic extrac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transition temperature | aaaaa | aaaaa |
| LCST | aaaaa | aaaaa |
| LCST | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com