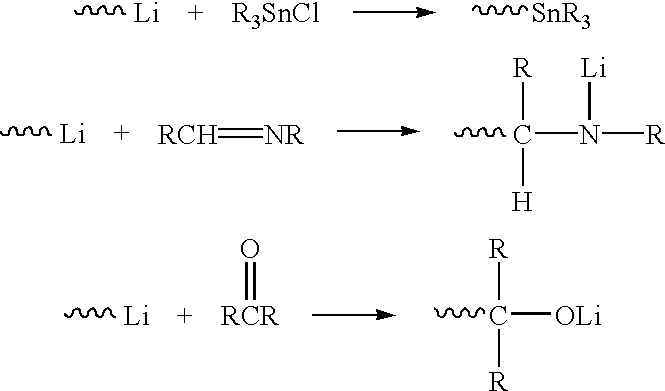
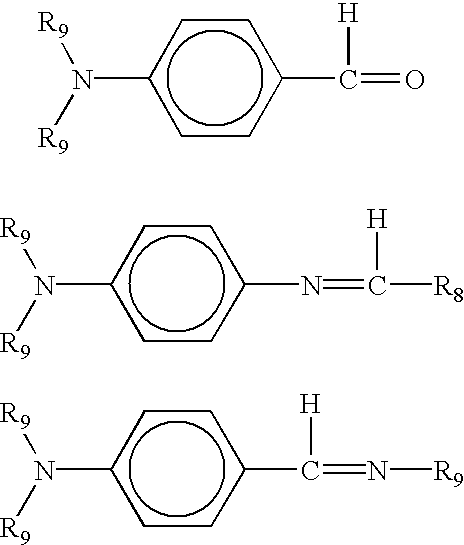
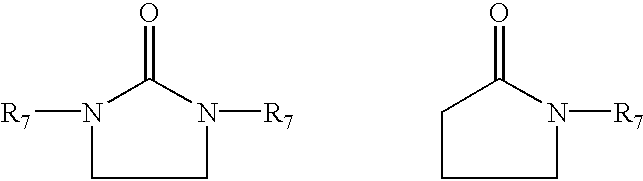Highly functionalized polymers and a process for making the same
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
[0055] Living polybutadiene polymers were prepared at low polymerization temperatures. These polymers were then divided into nine samples and each sample was subjected to different conditions. The polymers were then ultimately analyzed to determine whether the addition of lithium t-butoxide improved coupling with tin tetrachloride.
[0056] Specifically, polybutadienyllithium was prepared by admixing 3.21 Kg (7.07 lbs.) of a 25% by weight solution of 1,3-butadiene in hexane, 0.64 ml. of a 0.5 M solution of 2,2'-di (tetrahydrofuryl) propane in hexane, 5.75 ml. of a 1.0 M hexamethyleneimine in hexane, 3.90 ml. of a 1.63 M solution of n-butyllithium in hexane, and 1.71 Kg (3.76 lbs.) of dry hexane. This mixture was heated to about 49.degree. C. (120.degree. F.) for about 4 hours with agitation.
[0057] The resulting solution of polybutadienyllithium was divided and placed under pressure into nine capped and nitrogen-purged bottles. Three bottles were selected and subjected to three separate...
example ii
[0062] Two batches of polybutadiene polymers were prepared at high polymerization temperatures, subsequently subjected to heat treatment for various time intervals, and then coupled with tin tetrachloride. Lithium t-butoxide was added to one batch prior to the attainment of a peak polymerization temperature. The resulting polymers were analyzed for percent polymer conversion and percent coupling.
[0063] Specifically, the first batch was prepared by reacting 3.18 Kg (7.01 lbs.) of a 25 percent solution of 1,3-butadiene monomer in hexane, 0.43 ml of a 0.5 M 2,2'- di (tetrahydrofuryl) propane in hexane, 5.75 ml. of a 1.0 M hexamethyleneimine in hexane, 3.90 ml. of a 1.63 M solution of n-butyllithium in hexane, and 1.67 Kg (3.68 lbs.) of dry hexane. The reaction mixture was heated to 55.degree. C. (131.degree. F.) and a peak polymerization temperature of 90.5.degree. C. (195.degree. F.) was observed. Following this peak temperature, the reaction mixture was maintained at higher temperatu...
example iii
[0066] Two batches of styrene-butadiene copolymer were prepared at high polymerization temperatures. Lithium t-butoxide was added to the reaction mixtures at initial mixing. The polymers were coupled with tin tetrachloride and the percent polymer coupling was determined.
[0067] Specifically, a first batch of styrene-butadiene copolymer was prepared by charging the reactor with 549 grams (1.21 lbs.) of a 33.0% styrene solution in hexane, 2.12 Kg (4.67 lbs.) of 25.7% 1,3-butadiene solution in hexane, 7.8 ml. of 0.5 M 2,2'- di (tetrahydrofuryl) propane in hexane, 3.08 ml. of 1.95 M hexamethyleneimine in hexane, 3.80 ml. of 1.72 M solution of n-butyllithium in hexane, 6.4 ml. of 1.03 M solution of lithium t-butoxide in hexane, and an additional 1.75 Kg (3.85 lbs.) of dry hexane. This reaction mixture was heated by increasing the jacket temperature to about 117.degree. C. (242.degree. F.) until the batch temperature reached about 74.degree. C. (165.degree. F.). The mixture was then jacket...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com