Compounds and methods for delivery of prostacyclin analogs
a technology of prostacyclin and analogs, which is applied in the field of prostacyclin analogs, can solve the problems of ineffective administration of many valuable pharmacologically active compounds, potential local trauma to patients, and considerable discomfort to patients, and achieve the effect of increasing the oral bioavailability of treprostinil and the bioavailability of compound
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0196] In this Example, the bioavailability of treprostinil in rats after dosing orally, intraduodenally, intracolonically and via the portal vein was compared to determine possible barriers to bioavailability. In addition to bioavailability, a number of pharmacokinetic parameters were determined.
Animal Dosing
[0197] The bioavailability of treprostinil was evaluated in Sprague-Dawley, male rats. Fifteen surgically modified rats were purchased from Hilltop Lab Animals (Scottdale, Pa.). The animals were shipped from Hilltop to Absorption Systems' West Chester University (West Chester, Pa.), where they were housed for at least twenty-four hours prior to being used in the study. The animals were fasted for approximately 16 hours prior to dosing. The fifteen rats used in this study were divided into five groups (I, II, III, IV and V).
[0198] The weight of the animals and the dosing regimen are presented in Table 1.
TABLE 1DoseWeightRoute ofStudyVolumeDoseGroupRat #(g)AdministrationDay...
example 2
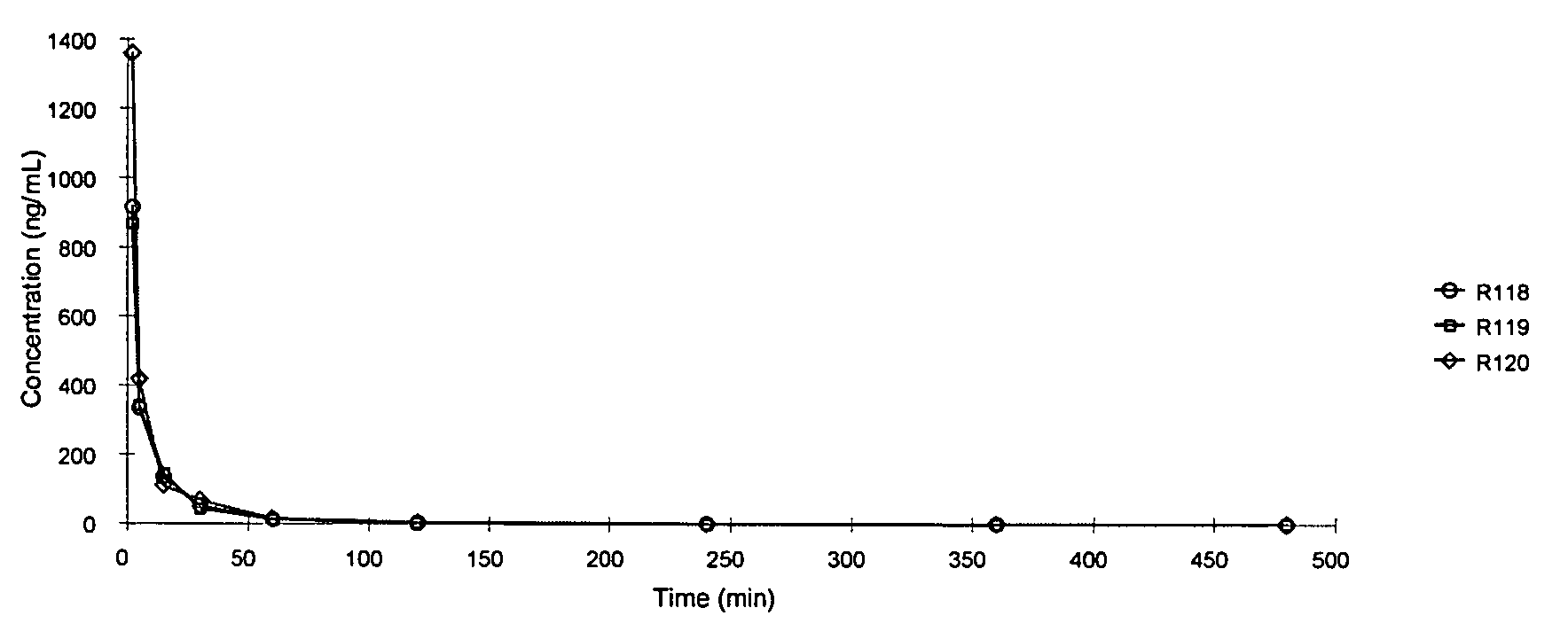
[0223] In this Example, Treprostinil concentrations were determined in male Sprague-Dawley rats following a single oral dose of the following compounds:
Experimental
Dosing Solution Preparation
[0224] All dosing vehicles were prepared less than 2 hours prior to dosing.
1. Treprostinil Methyl Ester
[0225] A solution of treprostinil methyl ester was prepared by dissolving 2.21 mg of treprostinil methyl ester with 0.85 mL of dimethylacetamide (DMA). This solution was then diluted with 7.65 mL of PEG 400:Polysorbate 80:Water, 40:1:49. The final concentration of the dosing vehicle was 0.26 mg / mL of treprostinil methyl ester equivalent to 0.25 mg / mL of Treprostinil. The dosing vehicle was a clear solution at the time of dosing.
2. Treprostinil Benzyl Ester
[0226] A solution of treprostinil benzyl ester was prepared by dissolving 2.58 mg of treprostinil benzyl ester with 0.84 mL of dimethylacetamide (DMA). This solution was then diluted with 7.54 mL of PEG 400:Polysorbate 80:Water, 40:...
example 3
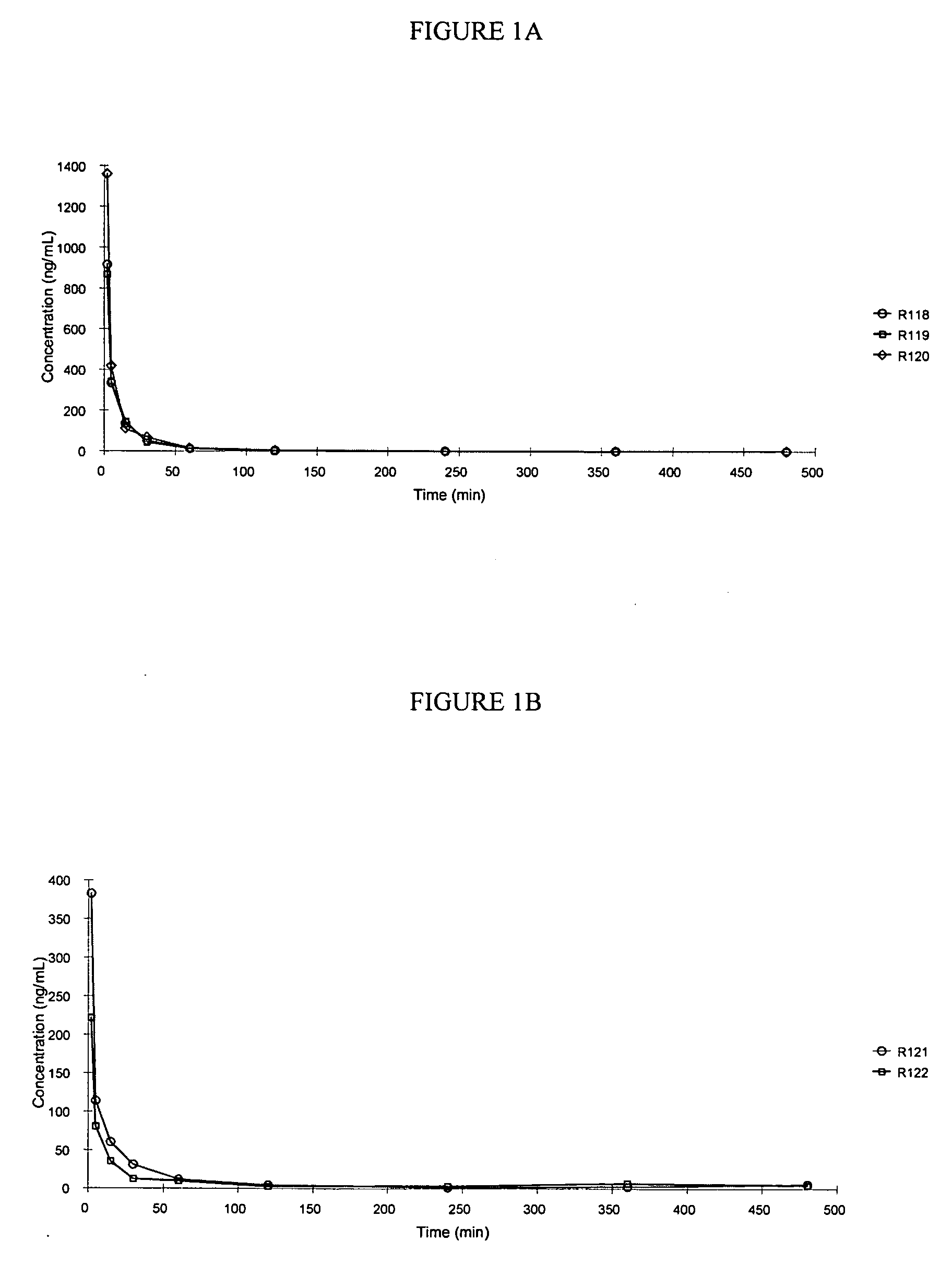
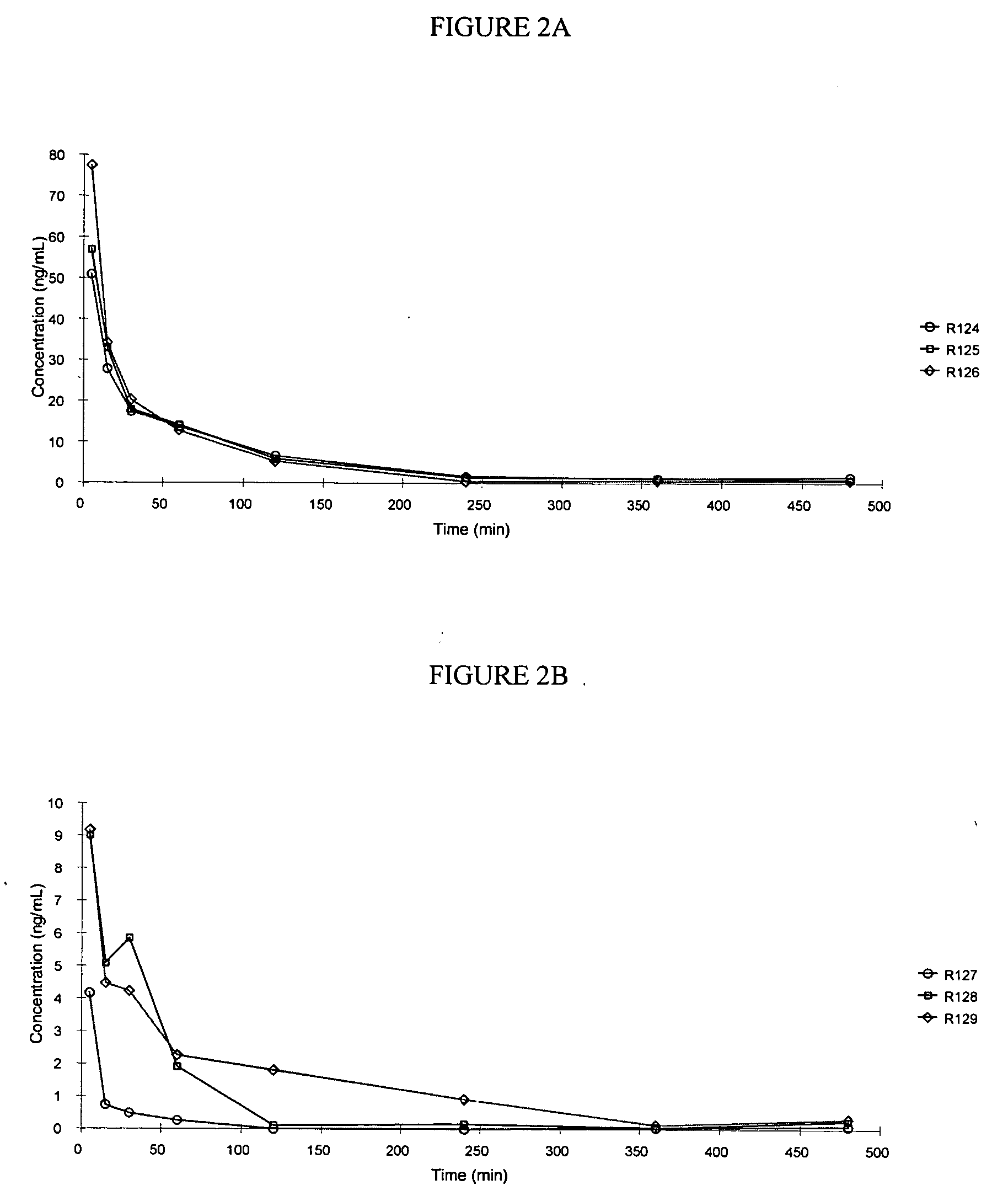
[0244] This example illustrates a pharmacokinetic study of treprostinil following administration of a single duodenal dose of treprostinil and various prodrugs of the present invention.
[0245] In this study, the area under the curve of Treprostinil in male Sprague-Dawley rats following a single intraduodenal dose of treprostinil monophosphate (ring), treprostinil monovaline (ring), treprostinil monoalanine (ring) or treprostinil monoalanine (chain), prodrugs of treprostinil was compared. The compounds were as follows:
[0246] having the following substituents:
CompoundR1R2R3treprostinilH—PO3H3Hmonophosphate(ring)treprostinilH—COCH(CH(CH3)2)NH2Hmonovaline(ring)treprostinilH—COCH(CH3)NH2Hmonoalanine(ring)treprostinilHH—COCH(CH3)NH2monoalanine(chain)
Experimental
Dosing Solution Preparation
[0247] All dosing vehicles were prepared less than 2 hours prior to dosing.
1. Treprostinil Monophosphate (Ring)
[0248] A dosing solution of treprostinil monophosphate (ring) was prepared by disso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bioavailability | aaaaa | aaaaa |
| Bioavailability | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com