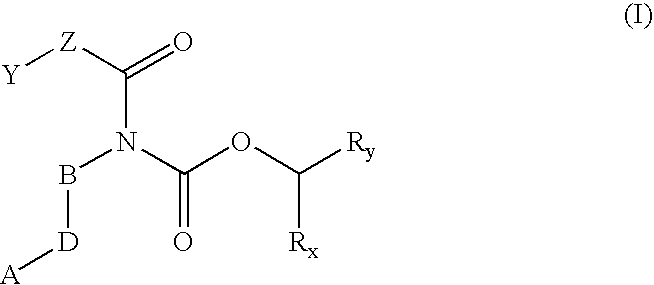
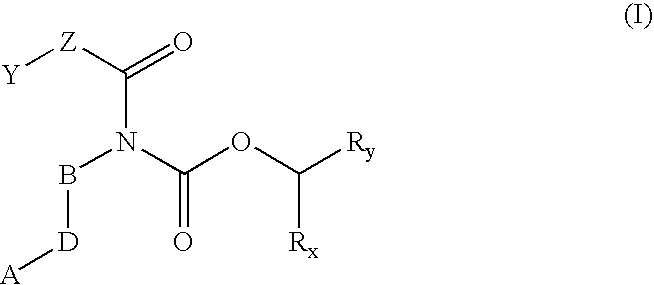
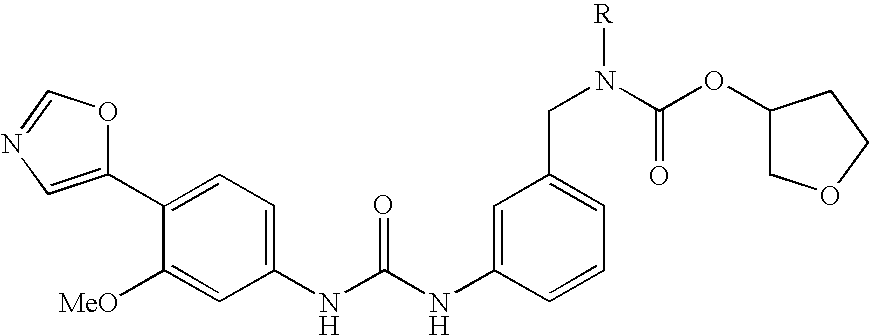
Prodrugs of carbamate inhibitors of IMPDH
a carbamate inhibitor and inhibitor technology, applied in the field of compound drugs, can solve the problems of poor bioavailability, undesirable pharmacological properties of mpa, gastrointestinal toxicity, etc., and achieve the effect of reducing the risk of gastrointestinal bleeding, preventing gastrointestinal bleeding, and ensuring the effect of gastrointestinal bleeding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0057] In order that the invention herein described may be more fully understood, the following detailed description is set forth. In the description, the following abbreviations are used:
DesignationReagent or FragmentEtethylBnbenzylDMFdimethylformamideDMSOdimethylsulfoxideEtOAcethyl acetateIPAisopropyl alcoholMHzmega-HertzNMRnuclear magnetic resonanceTFAtrifluoroacetic acidTHFtetrahydrofuran
[0058] The following terms are employed herein:
[0059] Unless expressly stated to the contrary, the terms “—SO2—” and “—S(O)2” as used herein refer to a sulfone or sulfone derivative (i.e., both appended groups linked to the S), and not a sulfinate ester.
[0060] The terms “halo” or “halogen” refer to a radical of fluorine, chlorine, bromine or iodine. The terms “immunosuppressant” and “immunosuppression agent” refer to a compound or drug which possesses immune response inhibitory activity. Examples of such agents include cyclosporin A, FK506, rapamycin, leflunomide, deoxyspergualin, prednisone...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pKa | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com