Methods of modulating lipid metabolism and storage
a technology of lipid metabolism and modulator, applied in the field of lipid metabolism and storage modulator, can solve the problems of no treatment for lipid metabolism disorders, and no treatment that can potentially eliminate the disorder
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Materials and Methods
1.1 Animal Housing and Treatment
[0261] Pregnant mice (C57BL / 6 or BALB / c) are either purchased (Jackson Laboratory, Bar Harbor, Me.) or matings set up in the mouse facility at Biogen, Inc. Pregnant mice at E12.5 of gestation are injected with 200 ug antibodies once every two days (6 mg / kg) by i.v. injection. The subsequently born offspring continue to receive injections (3 mg / kg) once every two days intraperitoneally (i.p.) until the time of sacrifice. In some experiments, mice receive injections only after birth (postnatal day 1) and followed the same injection regimens as the prenatal injections described above.
[0262] In experiments with adult mice that receive treatment only postnatally, two groups, each containing twenty (20) BALB / c mice (Jackson Labs, Bar Harbor Me.) were maintained under standard laboratory conditions. The two groups were divided into two main sections, dependent upon treatment. Group A mice are treated with hedgehog antagonist, while g...
example 2
Evaluation of Weight Loss in Mice Subject to the Modulating Effect of a Hedgehog Antagonist
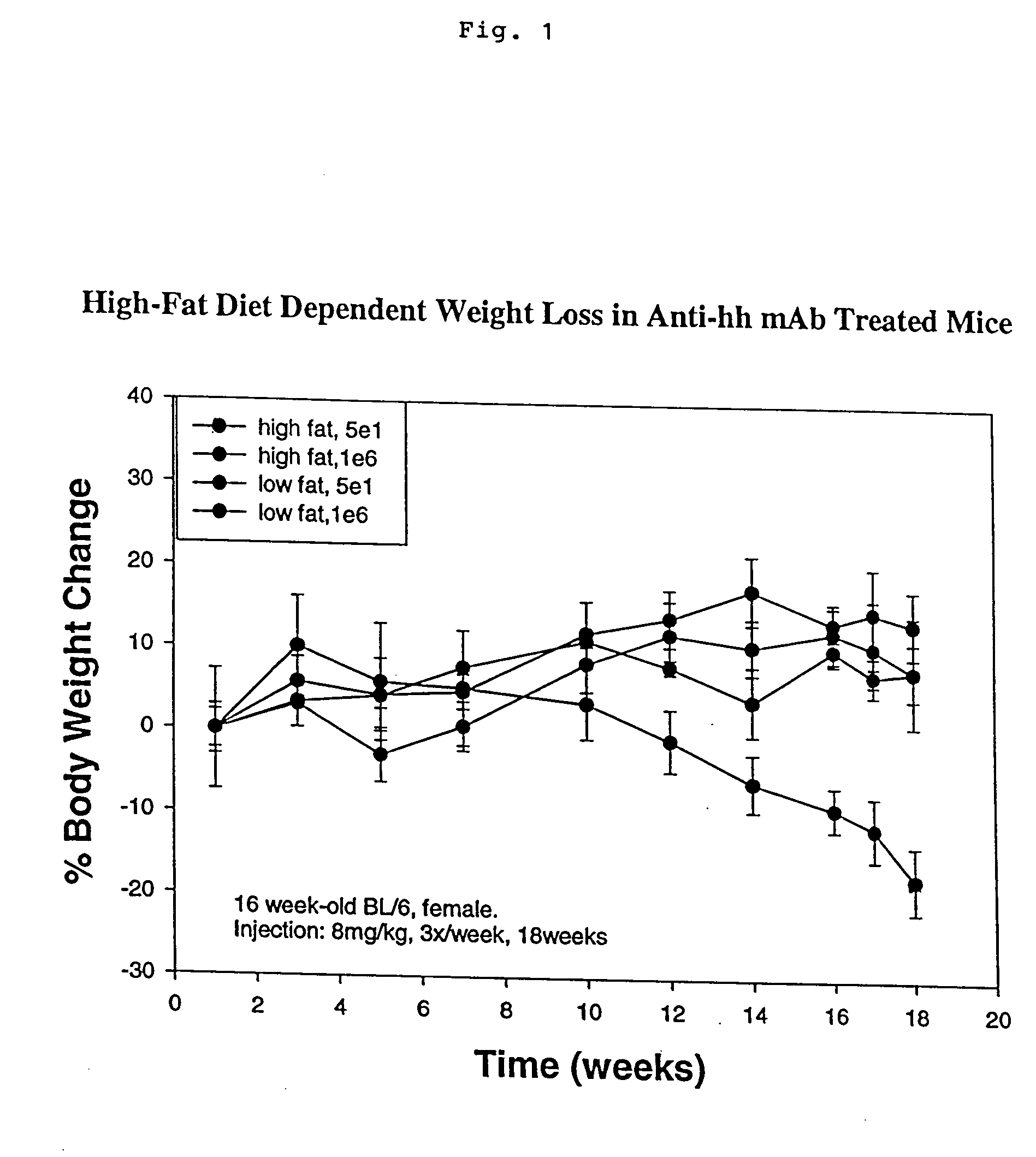
[0269] Three week old BALB / c mice (n=4) and 16 week old BL / 6 mice (n=4) are injected with control 1E6 mab or hedgehog antagonist 5E1 mab (8 mg / kg; three times per week) for 18 weeks. Mice are subject to either chow diet or high fat diet (19.2% fat) from the beginning of antibody treatments. Body weight is measured every week and is shown in FIG. 1 as a percentage of weight change as compared to the first weeks weight post treatment. There is a high-fat diet dependent weight loss only in the anti-hedgehog 5E1 treated group. Lipid accumulation (as evidenced by oil staining) was only observed in this group of mice.
example 3
Evaluation of the Modulating Effect of a Hedgehog Antagonist on Obese Mice
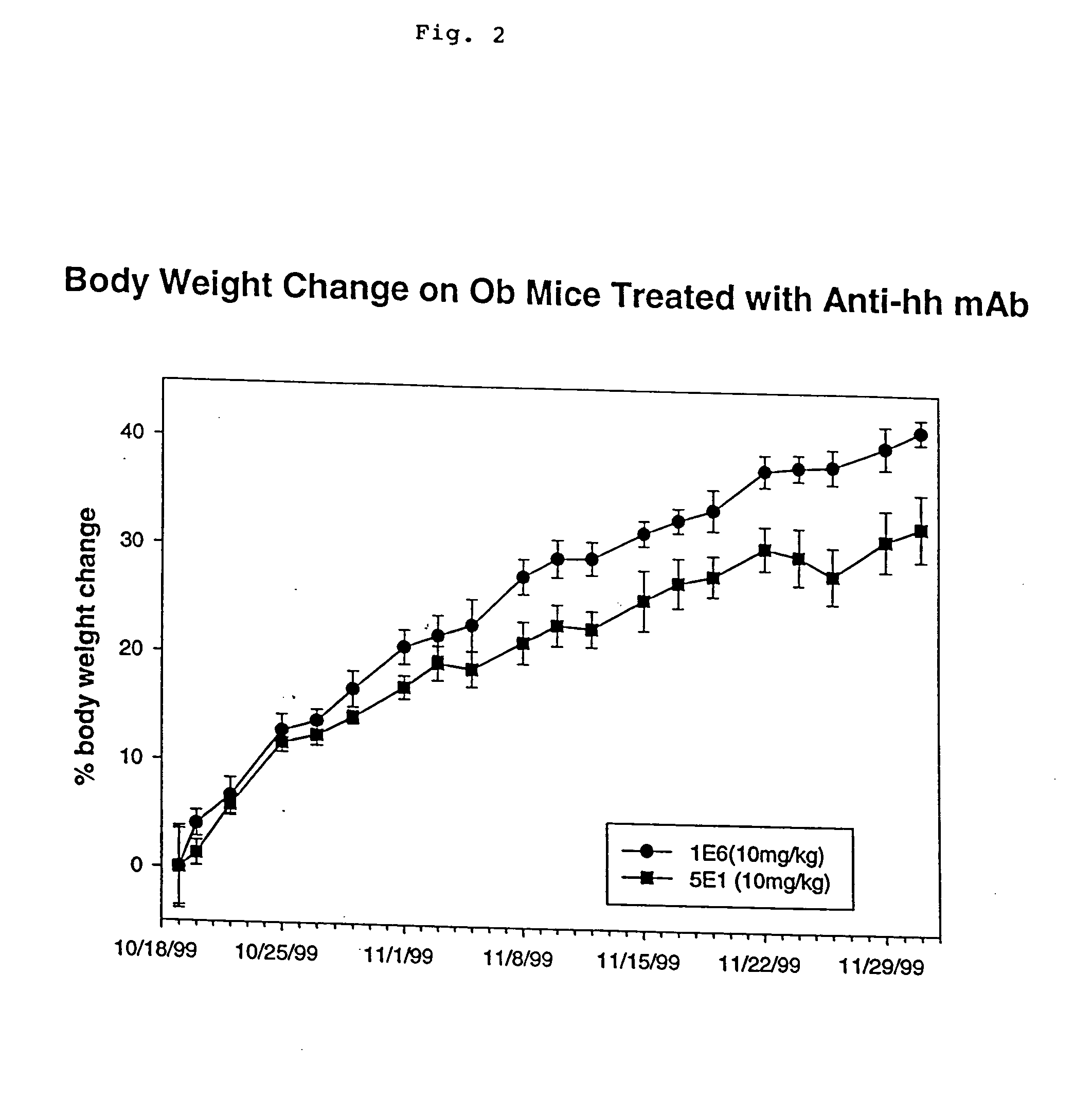
[0270] A strain of obese mice with a leptin gene mutation are obtained from Jackson Laboratory (Bar Harbor, Me.). Six week old obese mice are treated with 1E6 or 5E1 mabs (10 mg / kg; three times per week) for 8 weeks and the body weight measured before each injection (FIG. 2). The body weight is indicated as the percentage of weight change as compared to the original weight of the animal prior to the first injection. Treatment with hedgehog antagonist results in significant weight loss in the obese mice.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| apparent mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com