Novel method of diagnosing and treating gliomas
a glioma and glioma technology, applied in the field of cell physiology, neurology and neurooncology, can solve the problems of unsatisfactory, prior art deficient in lack of effective means, etc., and achieve the effects of short exposure time, reduced size of outward current, and reduced current amplitud
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Primary Cultures of Human Astrocytomas
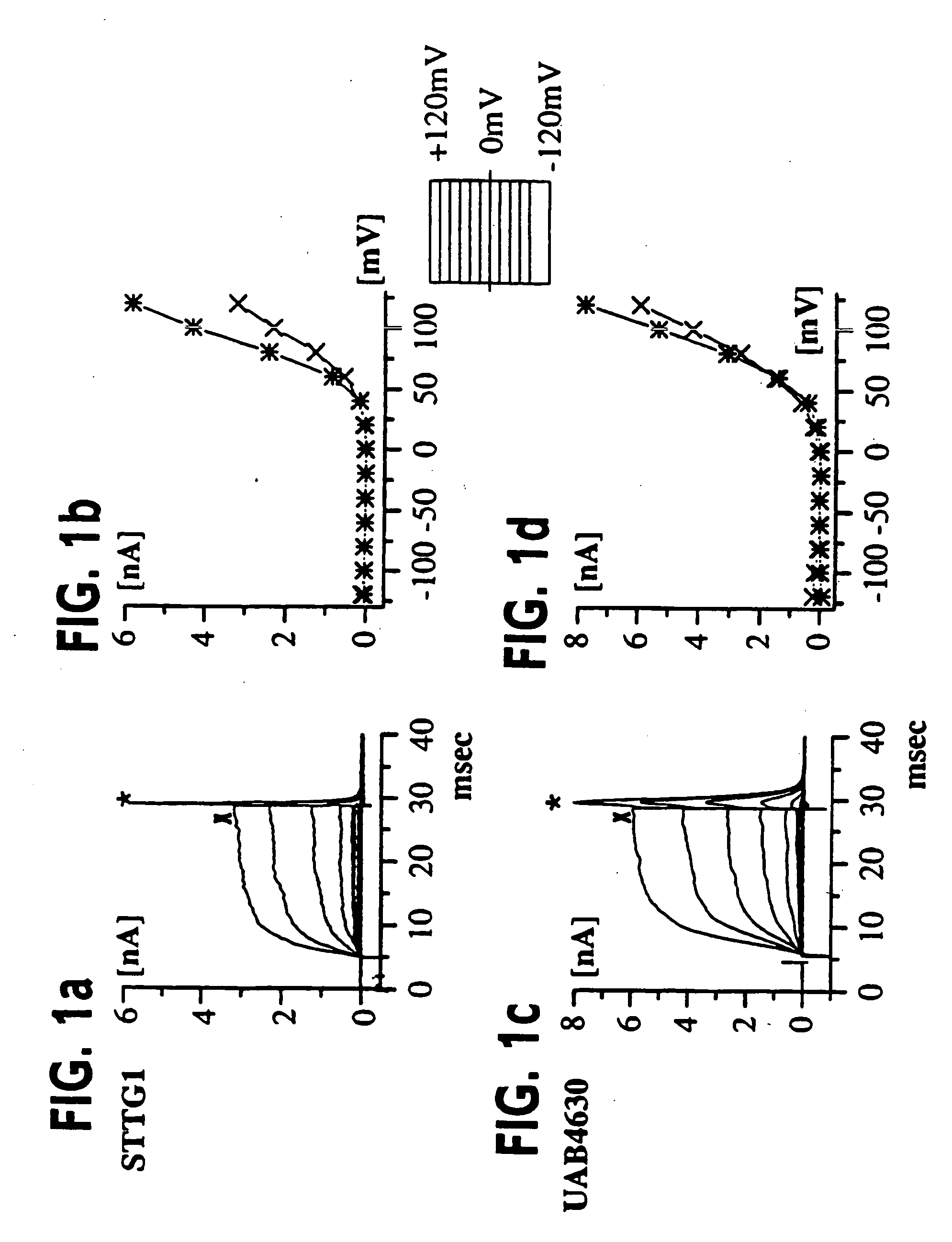
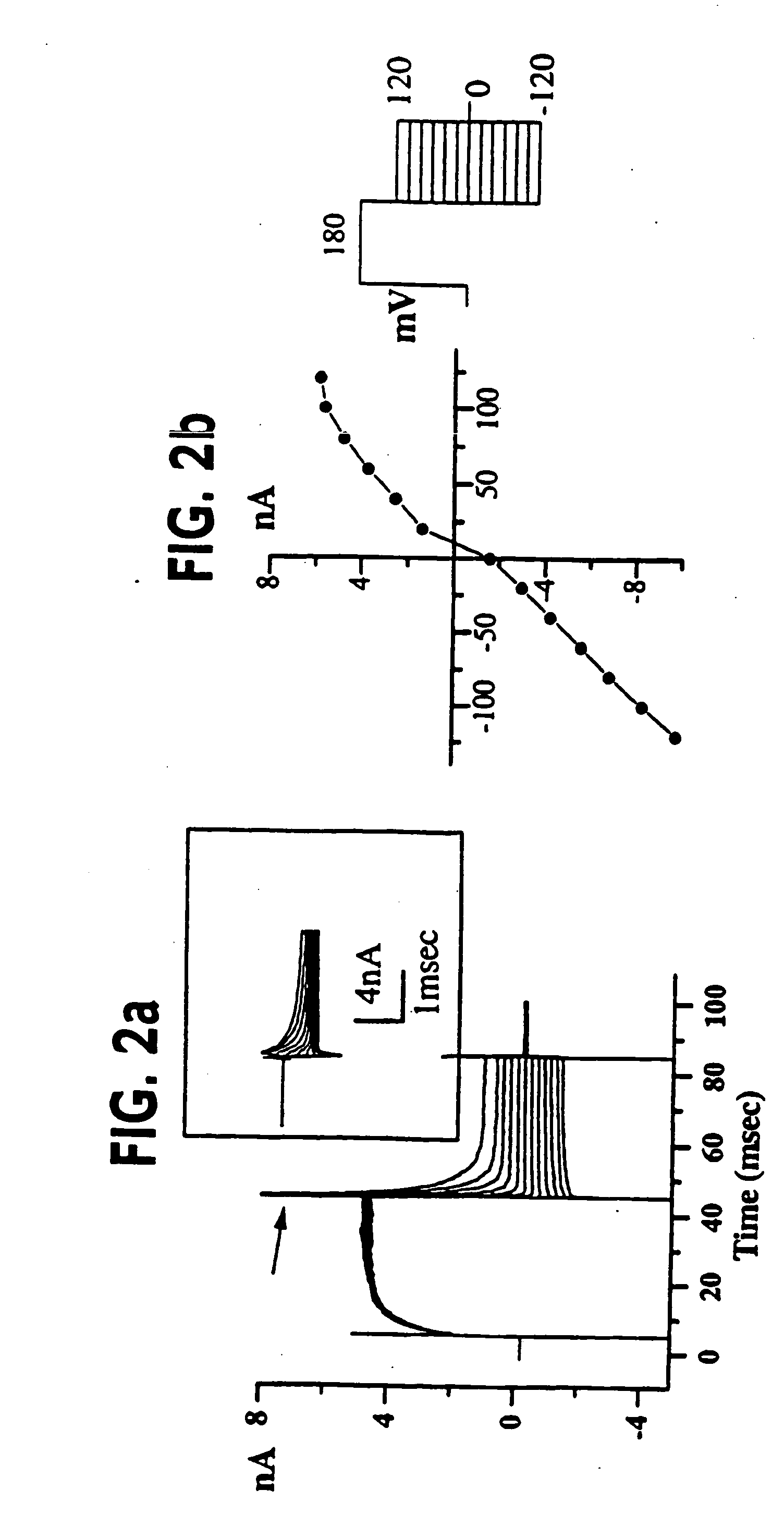
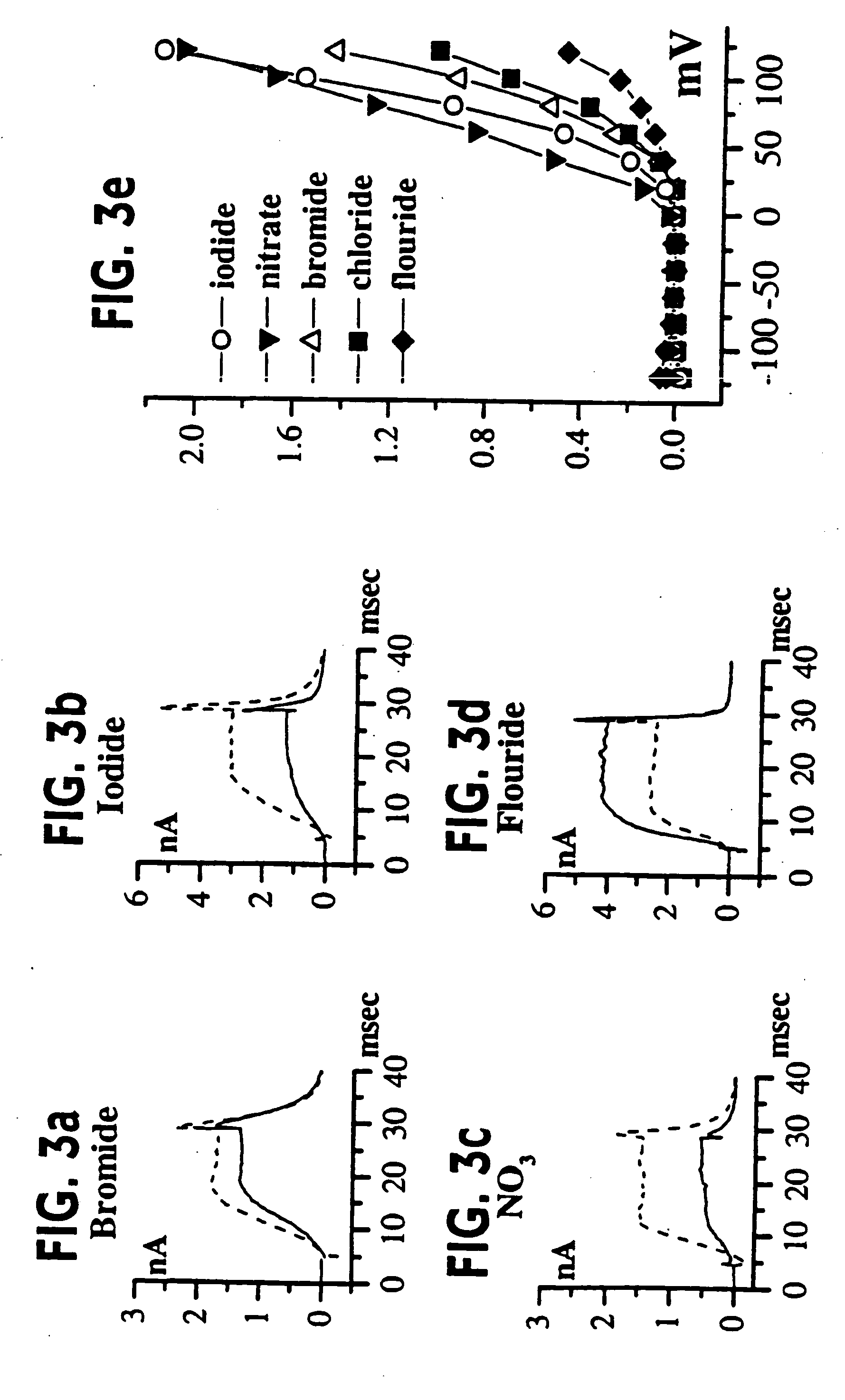
[0055] (UAB Brain Tumor Research Laboratories, see Table 1 for details): Freshly resected brain tumor tissue was transported in ice-cold tissue culture medium and necrotic / hemorrhagic portions were removed aseptically. Discrete pieces of tumor tissue were minced finely, triturated, and plated in DMEM / F12 (Dulbecco's modified Eagle's medium mixed equally with Ham's Nutrient Mixture F12 supplemented with 10 mM HEPES, 2 mM L-glutamine) with 20% Fetal Bovine Serum (FBS, Atlanta Biologicals). Cells from minced fragments were replated onto uncoated 12 mm round coverslips for electrophysiology and for GFAP immunocytochemistry. Acutely isolated tumor cells were prepared from fresh biopsy material, as described above with an additional trypsinization step in order to remove cellular debris, and were used for recordings 15-18 hours after plating.
example 2
Cell Lines
[0056] STTG1 cell line (American Type Culture Collection, Rockville, Md.) was grown in DMEM (Gibco) plus 10% FBS (Hyclone). Human Tumor Cell Lines: established cell lines, derived from human malignant gliomas (D54MG, U105MG, U251MG, and U373MG obtained from D. D. Bigner, Duke University) and extraglial human tumors (all from ATCC), were studied in long term (>100) passages (see TABLE I for details). Cells were maintained in DMEM / F12 supplemented with 7% heat-inactivated FBS (Atlanta Biologicals) at 37° C. in a 10% CO2 / 90% air atmosphere. Cells attaining nearly confluent growth were harvested and replated onto uncoated 75 cm2 flasks or uncoated 12 mm circular glass coverslips for electrophysiology and were used 36-72 hours after plating, unless otherwise noted. Viable cell counts were determined by trypan blue exclusion.
TABLE IPrimary cultures and established astrocytoma cell linesCell LineCl−DesignationCell TypePassageGFAPCurrentPrimary culturesUAB4630GBM1unk8 / 8UAB8553...
example 3
Biopsy Tissue
[0057] Freshly resected human brain tumor tissue are collected during surgery in ice-cold tissue culture medium and necrotic / hemorrhagic portions are removed aseptically. Tissue is maintained for 2 / CO2 until used. Ice-cold tissue are embedded in BactoAgar and cut into blocks of 10×10 mm and glued to the bottom of a petri dish mounted to a Vibratome where 200 mm slices are cut. These are transferred to oxygenated saline and maintained at 37° C. until recording.
PUM
| Property | Measurement | Unit |
|---|---|---|
| voltage | aaaaa | aaaaa |
| depolarizing voltage | aaaaa | aaaaa |
| morphology | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com