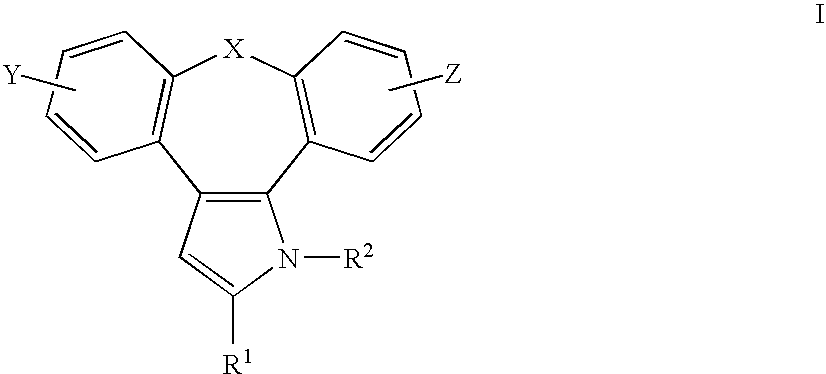
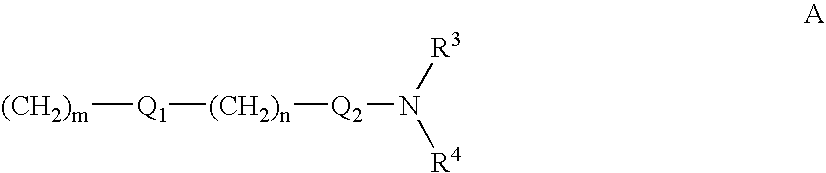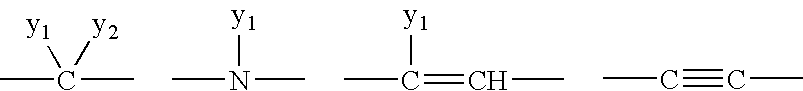1-aza-dibenzoazulenes as inhibitors of tumor necrosis factor production and intermediates for the preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 2
1H-8-Oxa-1-aza-dibenzo[e,h]azulene-2-carbaldehyde (7)
[0097] To dimethylformamide (38.7 mmol) cooled to 0° C., phosphoric trichloride (25.7 mmol) was added drop by drop and then the reaction mixture was stirred at room temperature for 15 minutes. To the reaction mixture cooled again to 0° C., a dimethylformamide solution of 1H-8-oxa-1-aza-dibenzo[e,h]azulene (4, 2.57 mmol in 5 mL) was added. Then the reaction mixture was stirred at 70-80° C. for 1-2 hours, cooled to room temperature and, by adding 50% NaOH, the pH was adjusted to 8-9. Such alkaline solution was heated for 1 hour at 70° C., then cooled to room temperature and poured into an ice-water mixture. The organic product was extracted with ethyl acetate, purified by chromatography on silica gel column and a yellow oily product was isolated.
[0098] According to the above process, by formylation of the compounds 5 and 6 there were prepared the compounds [0099] 11-chloro-1H-8-oxa-1-aza-dibenzo[e,h]azulene-2-carbaldehyde (8) and ...
example 3
1-(2-Trimethylsilyl-ethoxymethyl)-1H-8-oxa-1-aza-dibenzo[e,h]azulene-2-carbaldehyde (10)
[0100] A tetrahydrofuran solution of 1H-8-oxa-1-aza-dibenzo[e,h]azulene-2-carbaldehyde (7; 1.9 mmol in 15 mL) was cooled to 0° C. and slowly sodium hydride (60% dispersion in mineral oil, 125 mg) was added thereto. The reaction mixture was stirred at 0° C. until hydrogen stopped to develop (15-30 minutes) and trimethylsilyl ethoxymethyl chloride, (CH3)3SiCH2CH2OCH2Cl (SEM-Cl; 2 mmol) was added to the cooled reaction mixture. The reaction mixture was stirred at room temperature for one hour and then it was diluted by addition of water. The organic product was extracted with ethyl acetate. After drying the organic extracts on anhydrous sodium sulfate and evaporating the solvent, the crude product was purified by chromatography on a silica gel column. A dark oily product was isolated.
[0101] According to the above process, by silylating the compounds 8 and 9 there were prepared the compounds [0102]...
example 4
[1-(2-Trimethylsilyl-ethoxymethyl)-1H-8-oxa-1-aza-dibenzo[e,h]azulene-2-yl]-methanol (13)
[0104] To a methanolic solution of 1-(2-trimethylsilanyl-ethoxymethyl)-1H-8-oxa-1-aza-dibenzo[e,h]azulene-2-carbaldehyde (10; 2.45 mmol in 25 mL), NaBH4 (4 mmol) was added and the reaction mixture was stirred at room temperature for 2 hours. Then the pH of the reaction mixture was adjusted to 5 by adding acetic acid, the solvent was evaporated to dryness and the dry residue was extracted with ethyl acetate. By purifying the crude product by chromatography on a silica gel column an oily product was isolated.
[0105] According to the above process, by reacting the compounds 11 and 12 with NaBH4 there were prepared the compounds [0106] [11-chloro-1-(2-trimethylsilyl-ethoxymethyl)-1H-8-oxa-1-aza-dibenzo[e,h]azulene-2-yl]-methanol (14) and
[0107] [1-(2-trimethylsilyl-ethoxymethyl)-1H-8-thia-1-aza-dibenzo[e,h]azulene-2-yl]-methanol (15).
TABLE 1Icmp.XYZR1R2MS(m / z)1H NMR (ppm, CDCl3)4OHHHH231.96.59 (t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com