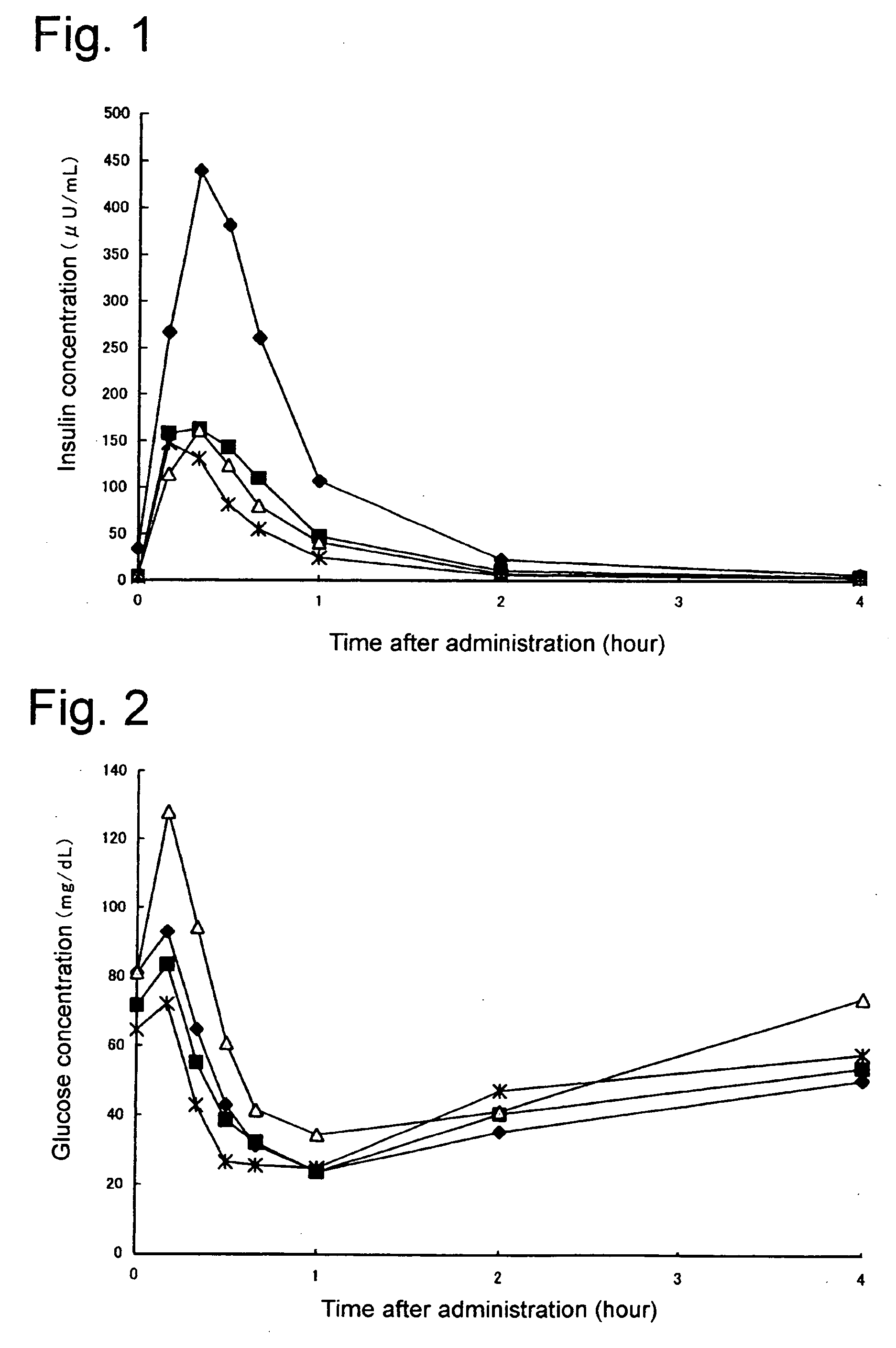
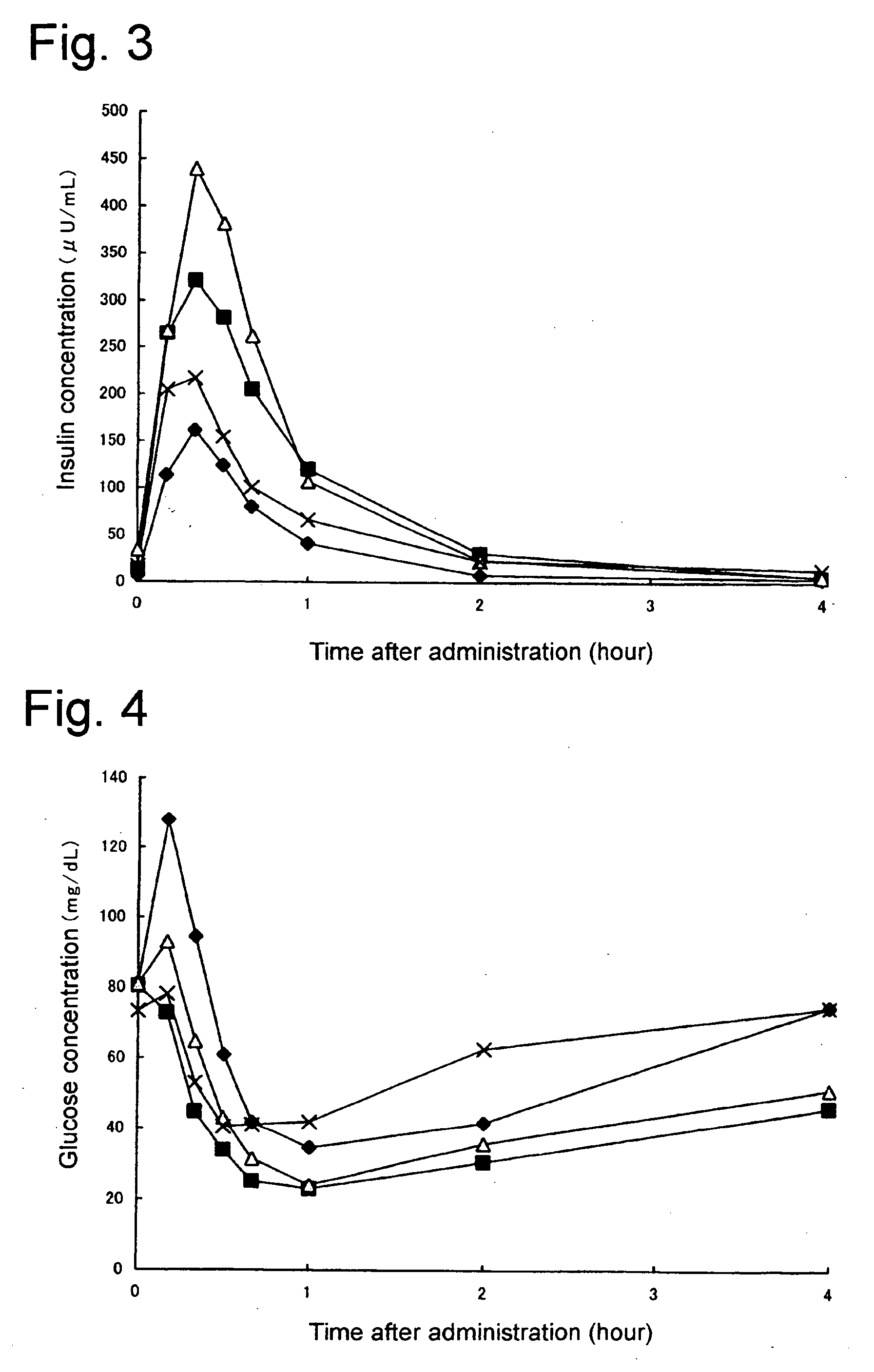
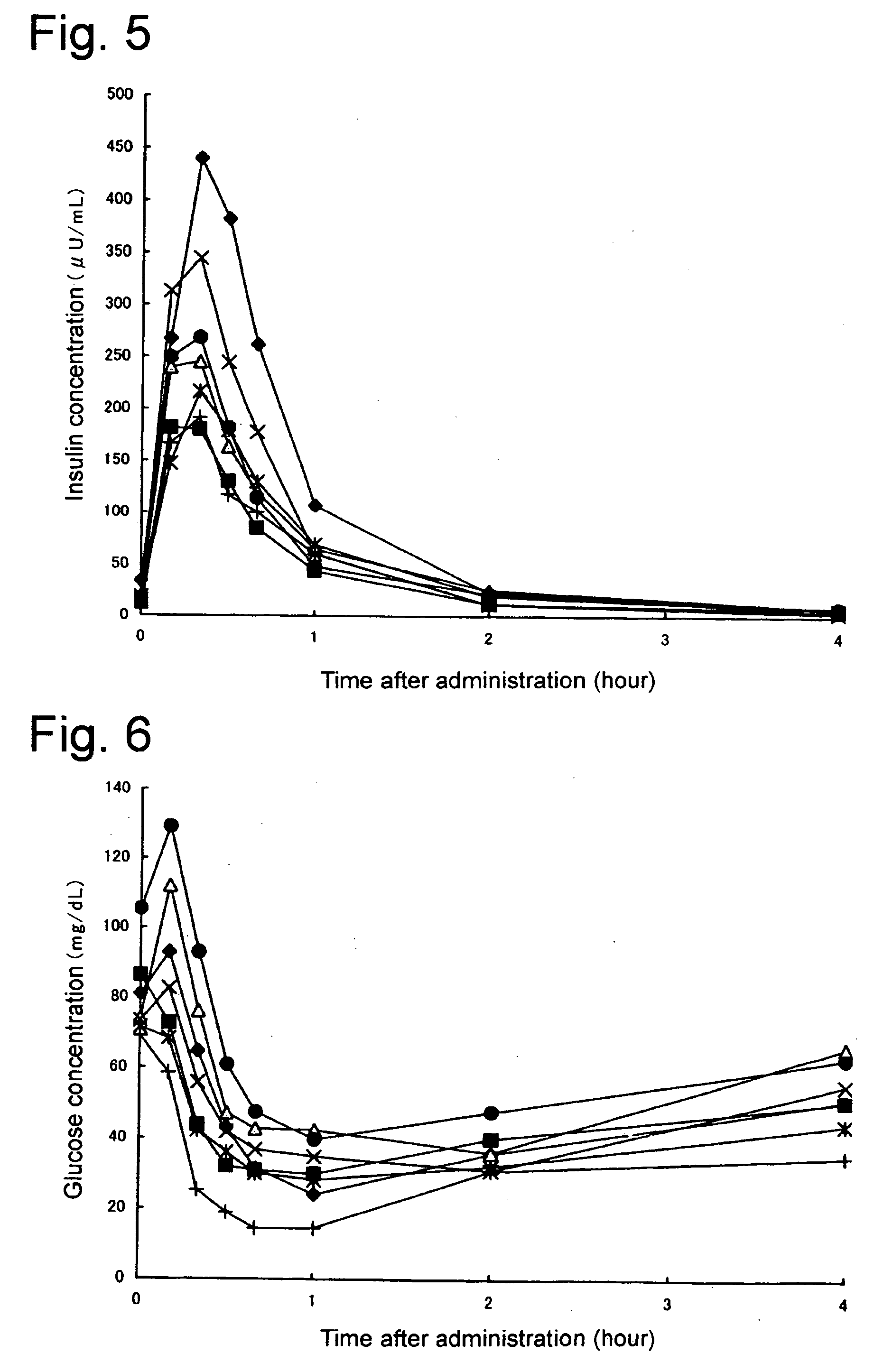
Composition of insulin for nasal administration
a technology for insulin and nasal administration, applied in the field of pharmaceutical formulations, can solve the problems of inability to disclose the practical application of insulin formulations for nasal administration including these related priors, and the inventors have no information on the practical application of insulin formulations for nasal administration, so as to improve the therapeutic effect of treatment and increase the absorption of insulin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0020] Any form of modified or unmodified insulin used in treating diabetes in humans can be used with the present invention, regardless of its origin. Accordingly, the term ‘Insulin’ as used here is deemed to mean all types in current or future clinical use, having the same physiologically activity as that of human insulin including human insulin, refined bovine insulin, refined porcine insulin, semi-synthesized human insulin, human isoinsulin, genetically modified human insulin or its variants thereof. Powder forms of these types of insulin or insulin that is only slightly soluble in water or almost insoluble in water (Corresponding to 1 g of powdered insulin requiring solvent in excess of 1000 mL and less than 10000 mL, or in excess of 10000 mL; see 13th Japan Pharmacopoeia, Principles of Operation A-51) even if modified, can also be preferably used with the present invention.
[0021] Given that the type of insulin described previously is slightly soluble in water or almost insolu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameters | aaaaa | aaaaa |
| particle diameters | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com