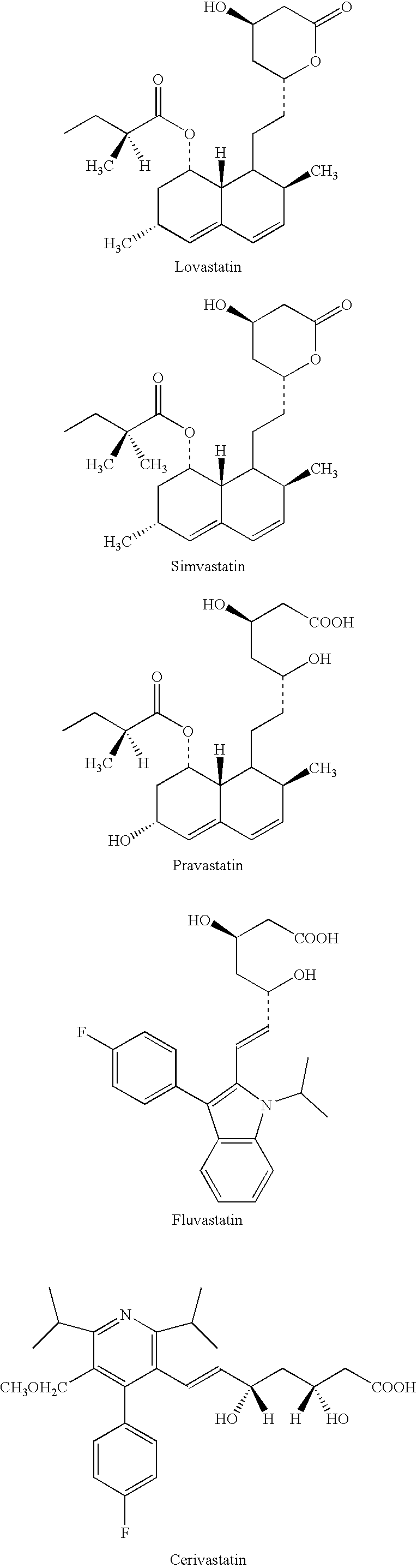
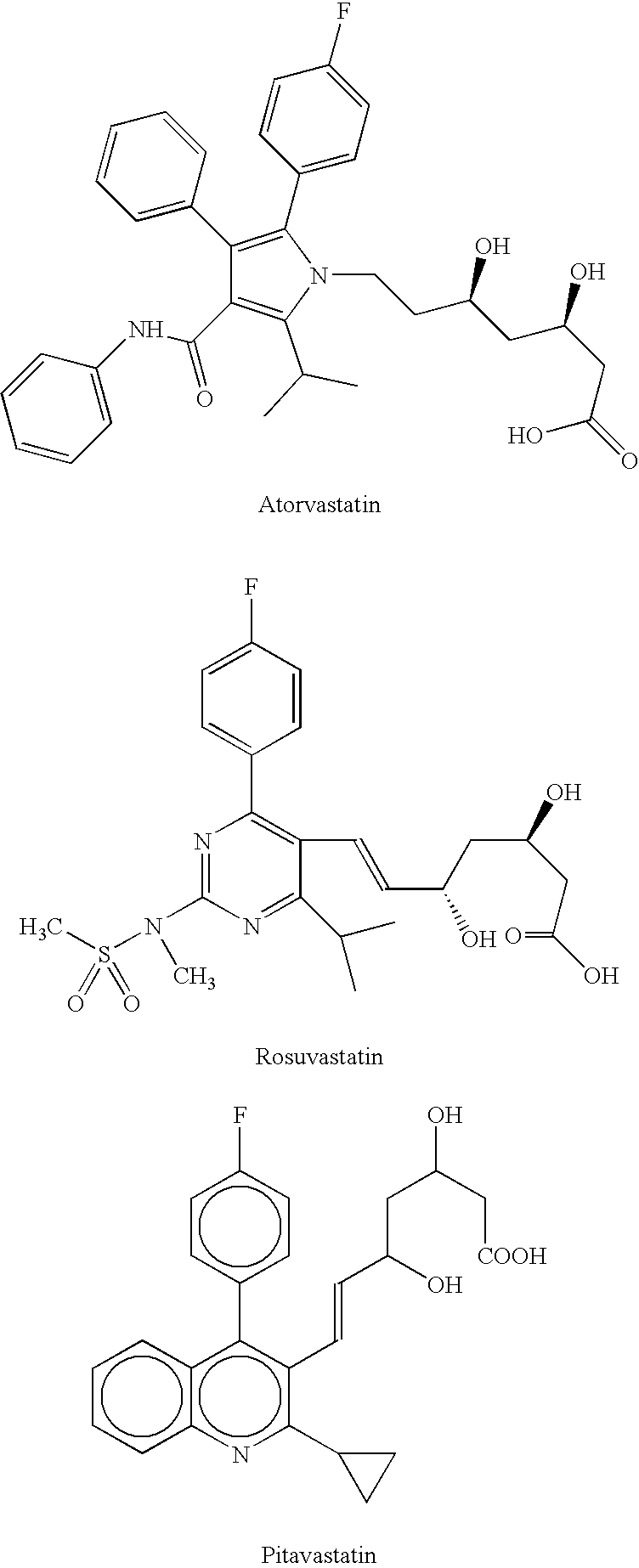
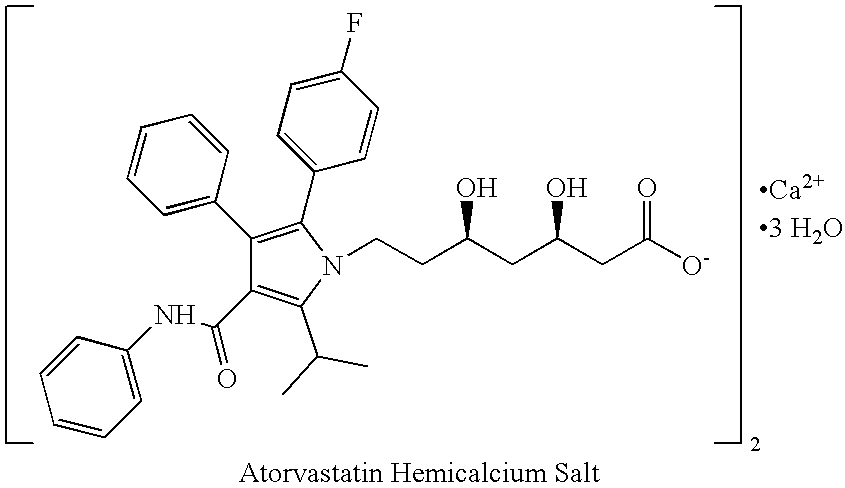
Processes for preparing calcium salt forms of statins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Atorvastatin Calcium from a Dioxane Ester Derivative
[0072] In a flask equipped with a magnetic stirrer, [R-(R*,R*)]-2-(4-fluorophenyl)-β,δ-dioxane-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-tert-butylheptanoic ester (2.0 g) was suspended in an 80% aqueous solution of acetic acid (50 ml). The mixture was stirred at ambient temperature for about 20 hours until a clear solution was obtained. The clear solution was evaporated to dryness and the traces of acetic acid were removed by azeotropic distillation with toluene (3×50 ml) to obtain a powder.
[0073] The above obtained powder (200 mg, 0.32 10−3 mole) was dissolved in ethanol (8 ml), to which a saturated solution of calcium hydroxide (8 ml) containing tetrabutyl ammonium bromide (10 mg) was added. The mixture was stirred and heated at a temperature of about 45° C. for about 24 hours. Additional saturated solution of calcium hydroxide (4 ml) was added. After about 20 minutes of stirring at ambien...
example 2
Preparation of Atorvastatin Calcium from a Dioxane Ester Derivative
[0074] In a flask equipped with a magnetic stirrer, [R-(R*,R*)]-2-(4-fluorophenyl)-β,δ-dioxane-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-tert-butylheptanoic ester (10.0 g, 15.29 10−3 mmole) was suspended in an 80% aqueous solution of acetic acid (150 ml). The mixture was stirred at ambient temperature overnight until a clear solution was obtained. The clear solution was evaporated and the traces of acetic acid were removed by azeotropic distillation with toluene (3×100 ml) to obtain an oily product containing toluene.
[0075] The oily product was placed in a mixture of ethanol (100 ml) and water (20 ml). A mixture of calcium hydroxide (5.5 eq., 6.22 g, 84.0 10−3 mmole) and 5% (w / w of the dioxane ester derivative) tetrabutyl ammonium bromide (0.46 g) was added. The mixture was heated to a temperature of about 45° C. for about 3 hours until the reaction was completed. While the mixture was hot, ...
example 3
Preparation of Atorvastatin Lactone from a Dioxane Ester Derivative
[0076] To a flask equipped with a magnetic stirrer, [R-(R*,R*)]-2-(4-fluorophenyl)-β,δ-dioxane-5-(1-methylethyl)-3-phenyl-4-[(phenylamino)carbonyl]-1H-pyrrole-1-tert-butylheptanoic ester (0.5 g, 0.76 10−3 mmole) was dissolved in a 1:1 mixture of trifluoroacetic-tetrahydrofuran (4 ml) in the presence of catalytic amount of water. The reaction mixture was stirred at ambient temperature for about 24 hours. The solution obtained was evaporated and the traces of trifluoroacetic were removed by azeotropic distillation with ether (3×100 ml). A white solid was obtained (0.3 g). Based on HPLC analysis, the white solid was a mixture of atorvastatin and atorvastatin lactone in the ratio of 40:60, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com