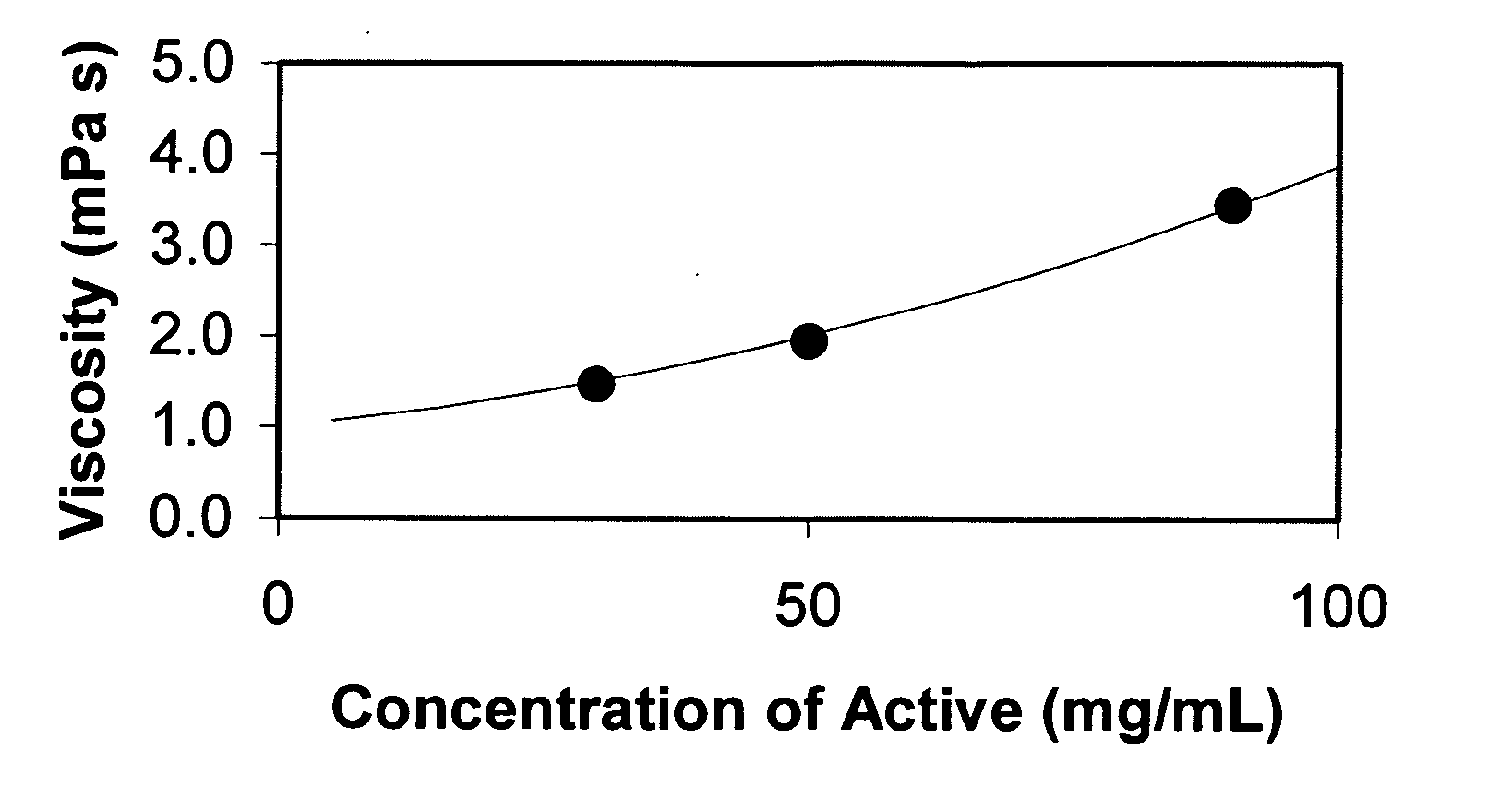
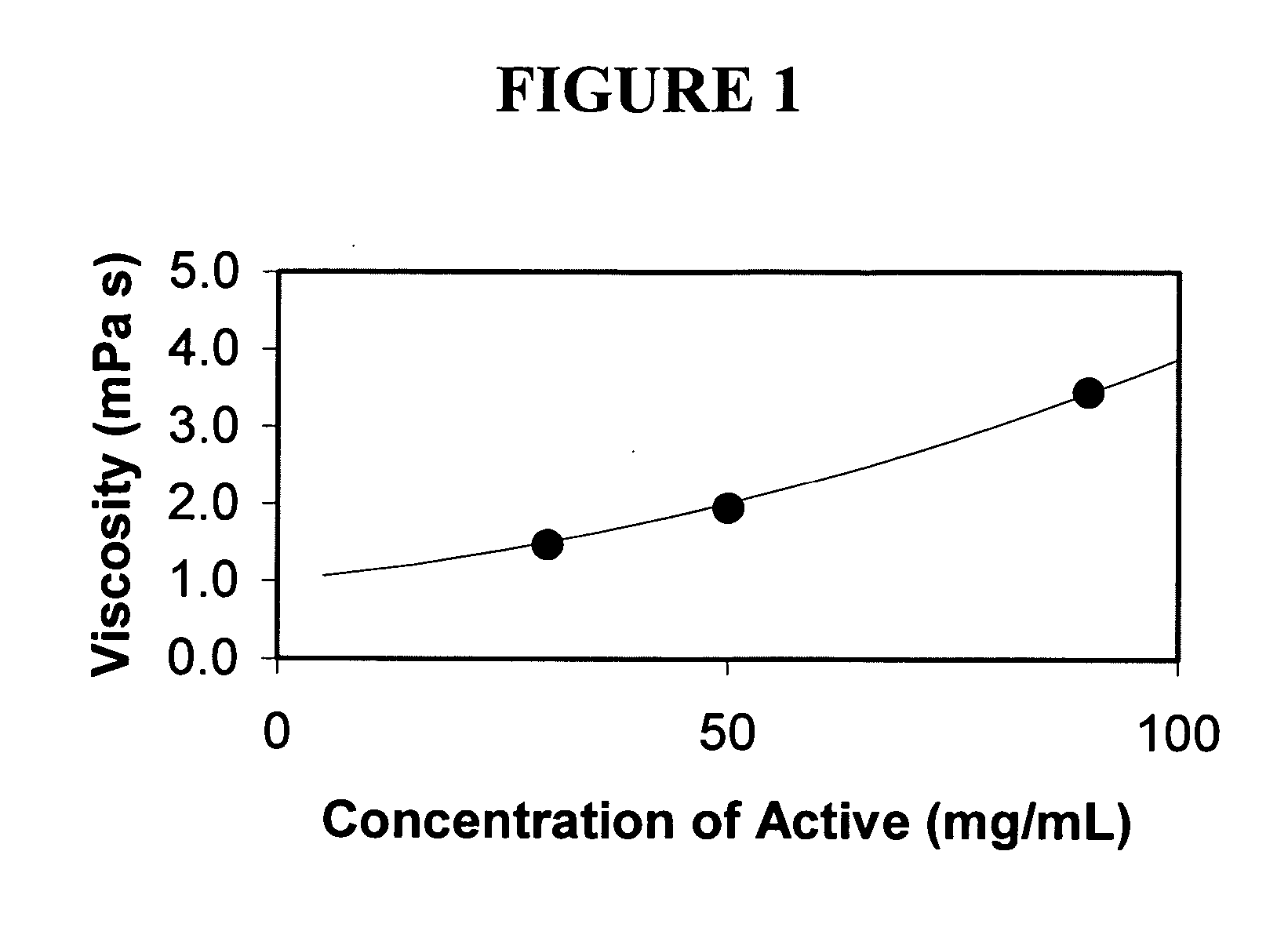
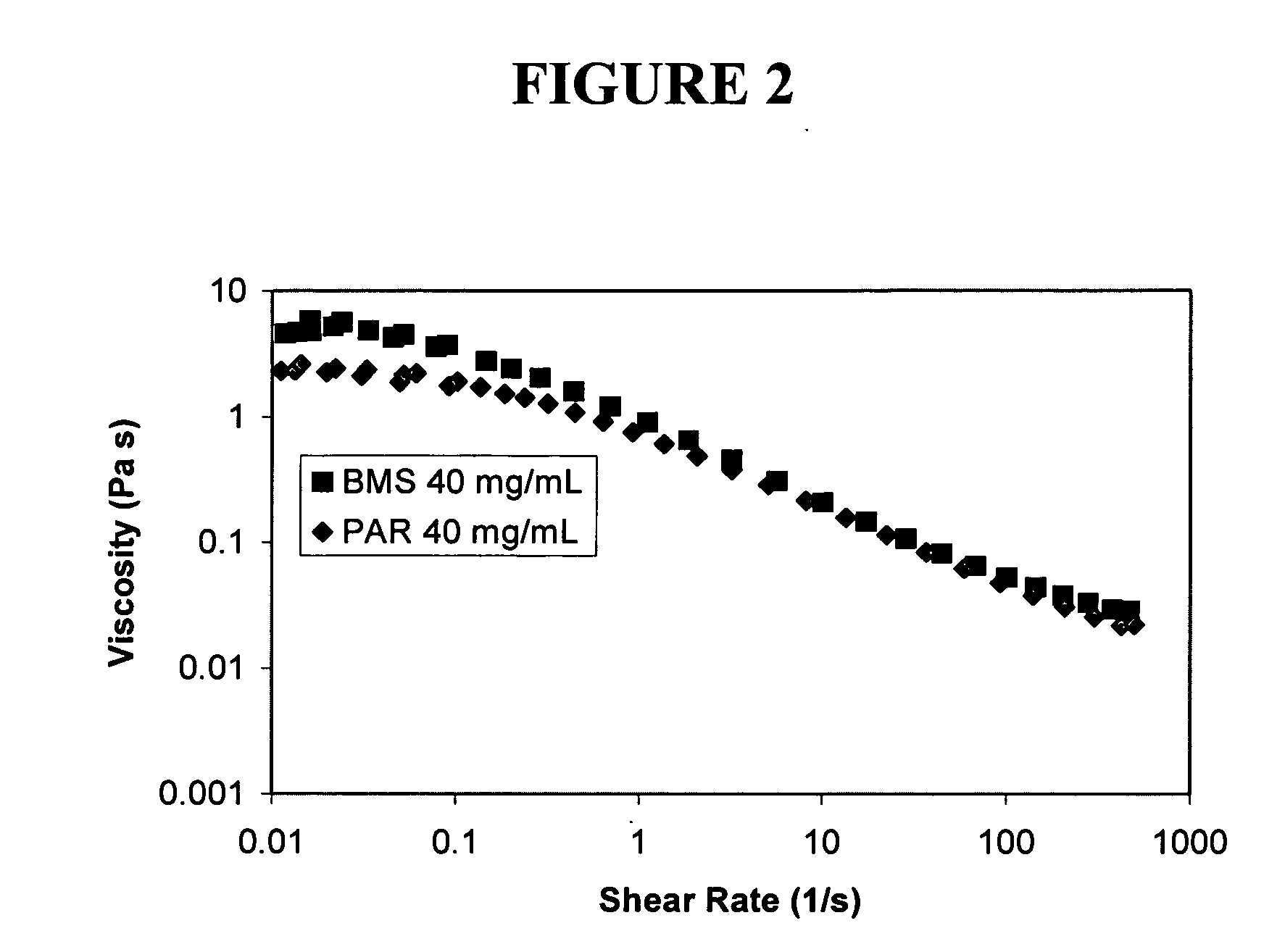
Nanoparticulate megestrol formulations
a technology of megestrol and nanoparticulate, which is applied in the direction of drug compositions, dispersed delivery, sexual disorders, etc., can solve the problems of unsatisfactory delivery system, high viscosity substances are not well accepted by patient populations, and relative long residence time, so as to improve physical (viscosity) and pharmacokinetic profiles, the effect of less variability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0187] The purpose of this example was to describe preparation of nanoparticulate dispersions of megestrol acetate.
[0188] Formulations 1, 2, 3, 4 and 5, shown in Table 1, were milled under high energy milling conditions using a NanoMill® (Elan Drug Delivery, Inc.) (see e.g., WO 00 / 72973 for “Small-Scale Mill and Method Thereof”) and a Dyno®-Mill (Willy Bachofen AG).
TABLE 1IdentityIdentity andandQuantityQuantityQuantityof Primaryof SecondaryofSurfaceSurfaceMeanD90FormulationMegestrolStabilizerStabilizer(nm)(nm)15%1% HPC-SL0.05% DOSS16722425%1% HPMC0.05% DOSS15621535%1% PVP0.05% DOSS16722645%1% Plasdone ®0.05% DOSS164222S630*55%1% HPMC0.05% SLS148208
*Plasdone ® S630 (ISP) is a random copolymer of vinyl acetate and vinyl pyrrolidone.
[0189] Formulations 1-5 showed small, well-dispersed particles using the Horiba La-910 Laser Scattering Particle Size Distribution Analyzer (Horiba Instruments, Irvine, Calif.) and light microscopy. Formulations 1-5 were stable in electrolyte fluids and...
example 2
[0190] This example compares the pharmacokinetic parameters of nanoparticulate megestrol acetate formulations of the present invention with conventional microparticulate formulations of megestrol acetate.
[0191] Twelve male beagles, at least twelve months of age, were divided into 2 groups based on whether they were fasting or being fed. The dogs were acclimated for thirteen days prior to dosing. The animals weighed approximately 11.4 to 14.3 kg at the time of dosing, and the dose was adjusted to 10 mg / kg. Water was available ad libitum. The animals were fasted (food only) for twelve to sixteen hours prior to dosing on day 1. On day 1, each dog was administered a formulation by gavage. Following dosing, the gavage tube was flushed with 18 ml of water. In the fed study, the animals were fed a high fat meal about 1 hour prior to dosing.
[0192] The dogs were subdivided into four groups, with each group receiving either Formulation-A (nanoparticulate megestrol dispersion #1, comprising ...
example 3
[0199] This example demonstrates the physical stability of megestrol acetate dispersions at various concentrations and with the addition of sucrose, flavoring, and preservatives.
[0200] Megestrol acetate was milled under high energy milling conditions using a NanoMill™2 System (Elan Drug Delivery, Inc.) in the presence of a preservative / buffer system consisting of sodium benzoate, citric acid monohydrate, and sodium citrate dihydrate. After milling, the resulting dispersion was diluted with water, sucrose, flavoring, and additional preservative / buffer to prepare dispersions containing 3% (w / w), 5% (w / w), or 9% (w / w) megestrol acetate. The resulting formulations are shown in Table 4. The physical stability of the formulations was then monitored at 25° C., 40° C., and 50° C.
TABLE 4Formulation SummaryConcentratedDiluted, Flavored DispersionsNanoparticleFormulation EFormulation FFormulation GDispersion3% Dispersion5% Dispersion9% DispersionAPI and Excipientsg / kgg / kgg / kgg / kgMegestrol A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com