Isoprenoid pathway inhibitors for stimulating cartilage growth
a technology of isoprenoid pathway and inhibitor, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of severe limitations to the development of bmps as therapeutic agents, limiting their usefulness as therapeutic agents when administered systemically, and affecting the effect of bone healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
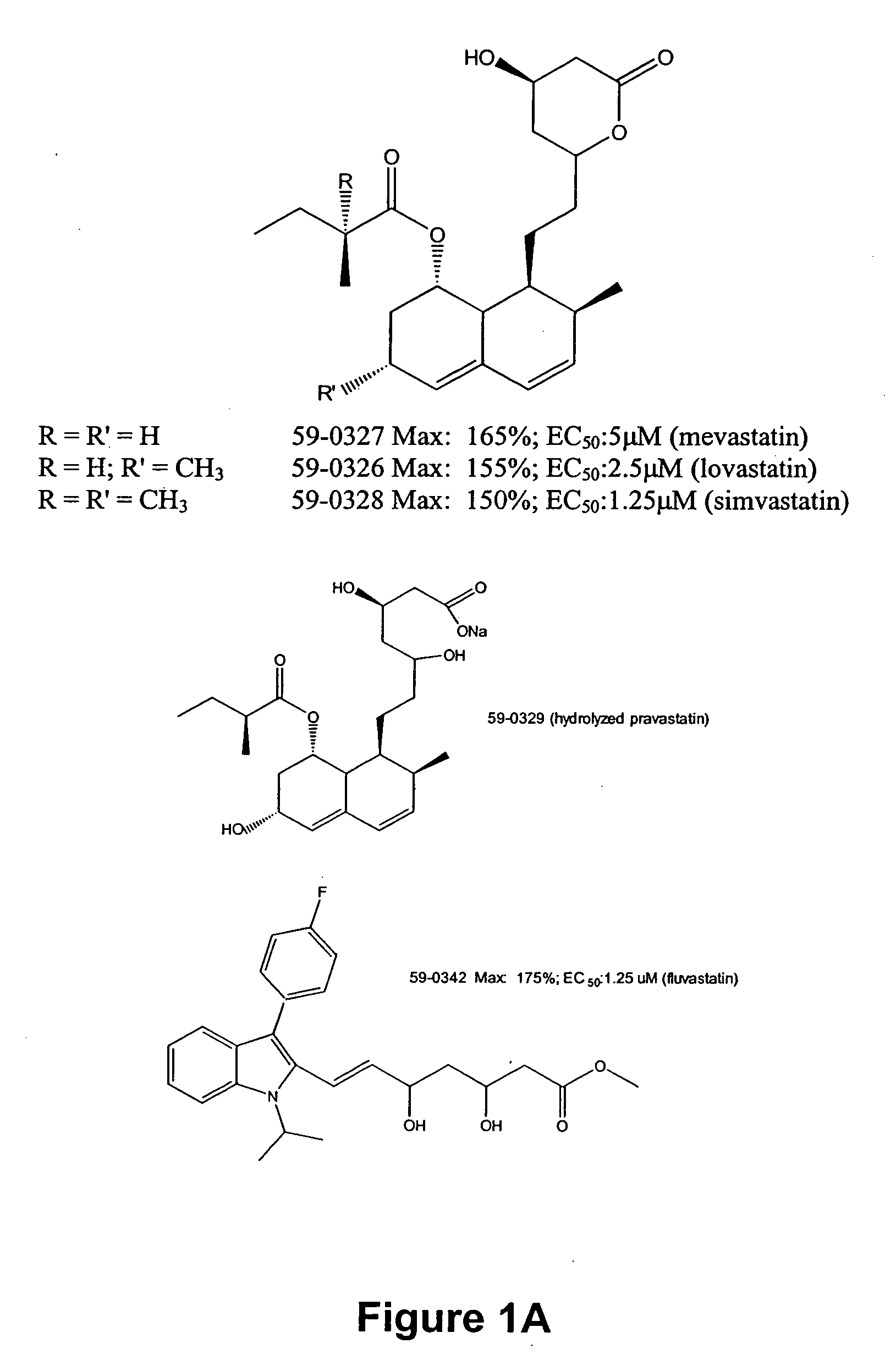
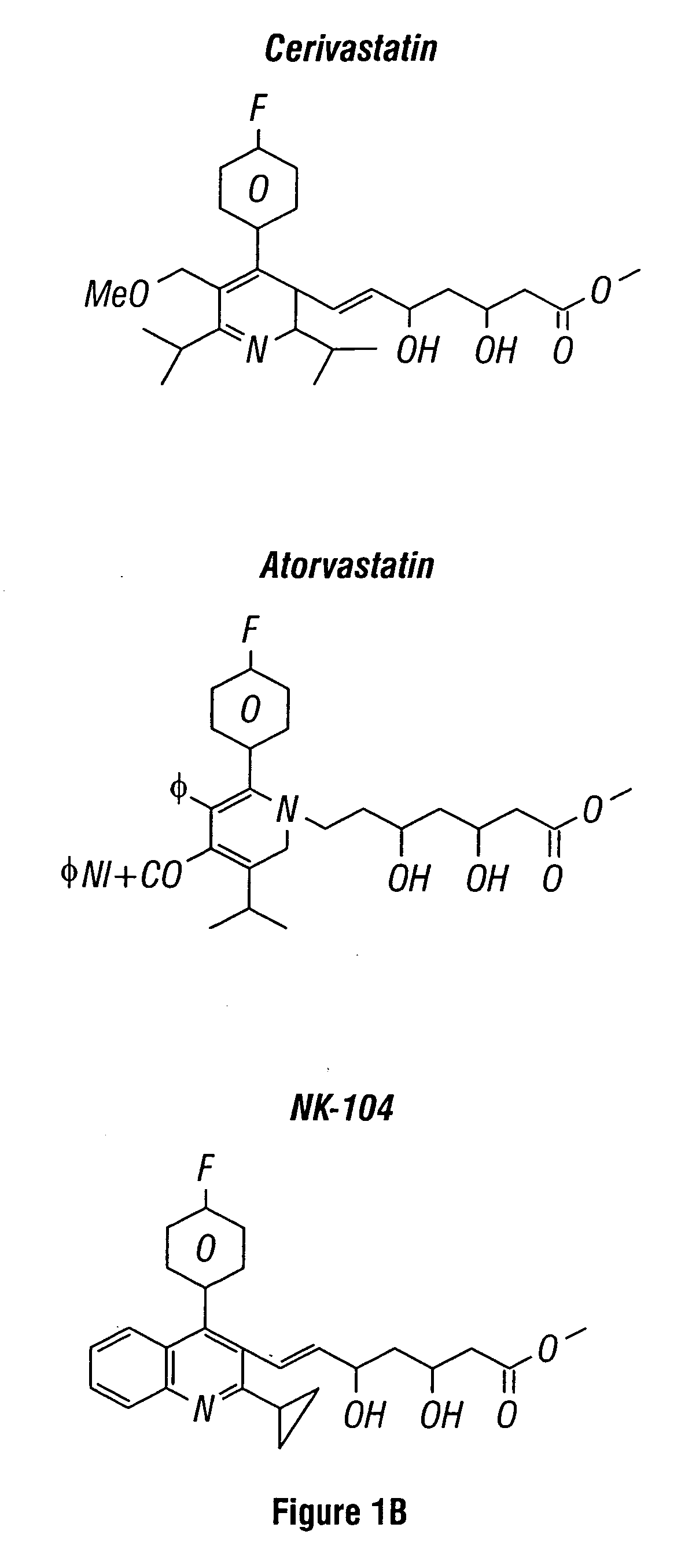
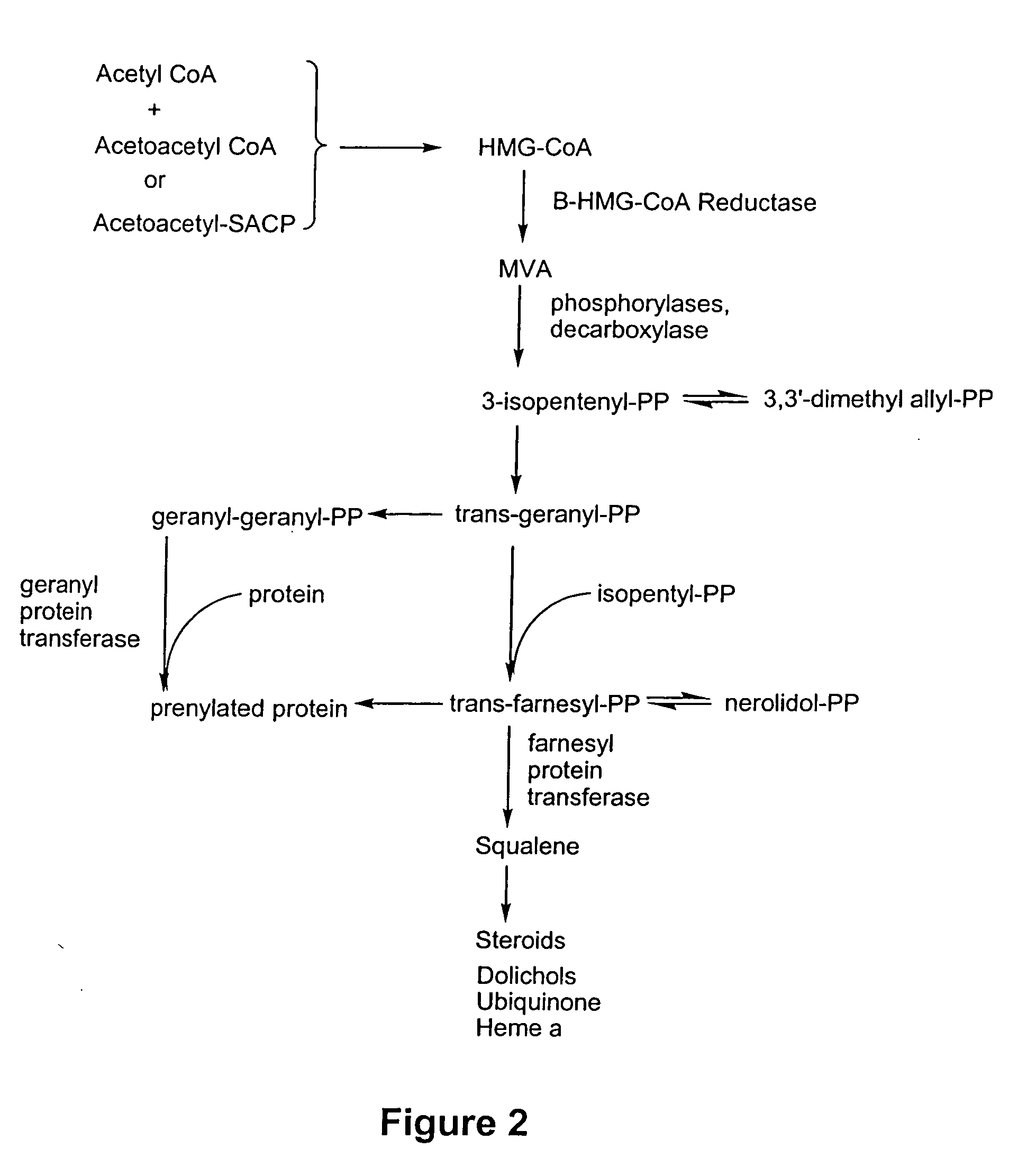
[0109] Thousands of compounds have been tested in the assay system set forth in U.S. Ser. No. 08 / 458,434, filed 2 Jun. 1995, and incorporated herein by reference. Representative compounds of the invention gave positive responses, while the majority of (unrelated) compounds are inactive. In this screen, the standard positive control was the compound 59-0008 (also denoted “OS8”), which is of the formula:
[0110] In more detail, the 2T3-BMP-2-LUC cells, a stably transformed osteoblast cell line described in Ghosh-Choudhury, et al., Endocrinology (1996) 137:331-39, referenced above, was employed. The cells were cultured using α-MEM, 10% FCS with 1% penicillin / streptomycin and 1% glutamine (“plating medium”), and were split 1:5 once per week. For the assay, the cells were resuspended in a plating medium containing 4% FCS, plated in microtiter plates at a concentration of 5×103 cells (in 50 μl) / well, and incubated for 24 hours at 37° C. in 5% CO2. To initiate the...
example 2
In Vivo Calvarial Bone Growth Data
[0116] Lovastatin and simvastatin were assayed in vivo according to the procedure described previously (see “In vivo Assay of Effects of Compounds on Murine Calvarial Bone Growth,” supra). Simvastatin provided a 1.5 fold increase in the number of osteoblasts.
[0117] In one experiment, vehicle control, bFGF and varying doses of simvastatin (59-0328) and lovastatin (designated 59-0326) were tested in the in vivo calvarial bone growth assay. The results are reported as a measurement of total bone area (and % increase in area over vehicle control), as shown below.
Total BoneCompoundArea (μm2)Control167.7bFGF (12.5 μg / kg / day)242 (45%)59-0328 10 mg / kg / day245 (46%)59-0328 5 mg / kg / day202 (20%)59-0328 1 mg / kg / day172 (2%)59-0326 10 mg / kg / day239 (42%)59-0326 5 mg / kg / day235 (40%)59-0326 1 mg / kg / day237 (41%)59-0326 0.1 mg / kg / day162 (0%)
[0118] Both simvastatin and lovastatin stimulated a dose-dependent increase in total bone area. At 10 mg / kg / day, the bone stim...
example 3
[0119] Selected compounds and appropriate controls were assayed in vitro (ex vivo) for bone formation activity (described above in “Neonatal Mouse Calvaria Assay (in vitro)”). Histomorphometrical assessments of ex vivo calvaria were carried out using an OsteoMetrics bone morphometry measurement program, according to the manufacturer's instructions. Measurements were determined using either a 10- or 20-fold objective with a standard point counting eyepiece graticule.
[0120] New bone formation was determined (using a 10×objective) by measuring the new bone area formed in one field in 3 representative sections of each bone (4 bones per group). Each measurement was carried out ½ field distance from the end of the suture. Both total bone and old bone area were measured. Data were expressed as new bone area in mm2.
[0121] Osteoblast numbers were determined by point counting. The number of osteoblast cells lining the bone surface on both sides of the bone were count...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
| Electric charge | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com