Nasal spray formulation and method
a nasal spray and formulation technology, applied in the direction of aerosol delivery, drug compositions, peptide/protein ingredients, etc., can solve the problems of limiting the success of nasal spray formulations, low bioavailability, mucosal irritation, etc., to prevent signs and symptoms of estrogen deficiency, suppress ovarian estrogen and progesterone production, and prevent bone mineral density loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
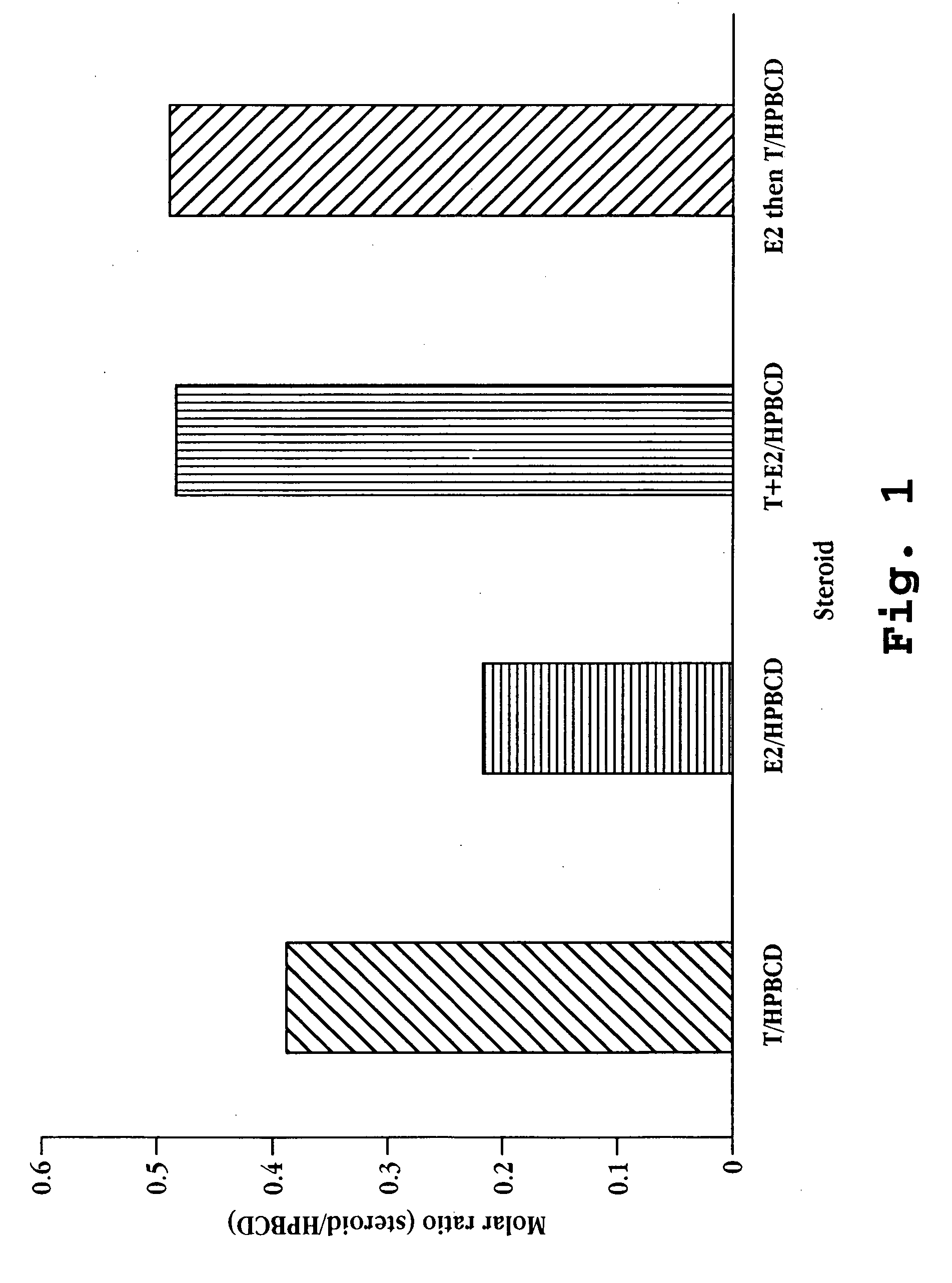
Solubility of Estradiol and Testosterone in Cyclodextrin / Water
[0091] The solubility of 17-β-estradiol and testosterone in varying concentrations of 2-hydroxypropyl-β-cyclodextrin (MW 1380 g / mole; 5.5 degree of substitution) was determined as follows. 10 ng 17-β-estradiol (MW 272.39 g / mole) was added to 1 mL of 2-hydroxypropyl-β-cyclodextrin in water, the 2-hydroxypropyl-β-cyclodextrin concentration ranging from 10 to 250 ng / mL. In a second series of vials, 20 ng of testosterone (MW 288.43 g / mole) was added to 1 mL of 2-hydroxypropyl-β-cyclodextrin in water, the 2-hydroxypropyl-β-cyclodextrin concentration ranging from 10 to 250 ng / mL. In a third set of vials 10 ng 17-β-estradiol and 20 ng testosterone were added to 1 mL of 2-hydroxypropyl-β-cyclodextrin in water, the 2-hydroxypropyl-β-cyclodextrin concentration ranging from 10 to 250 ng / mL. The vials were mixed at room temperature for about 1 hour. Aliquots were taken from the supematant of each vial and assayed for steroid concent...
example 2
Preparation of Intranasal Formulation
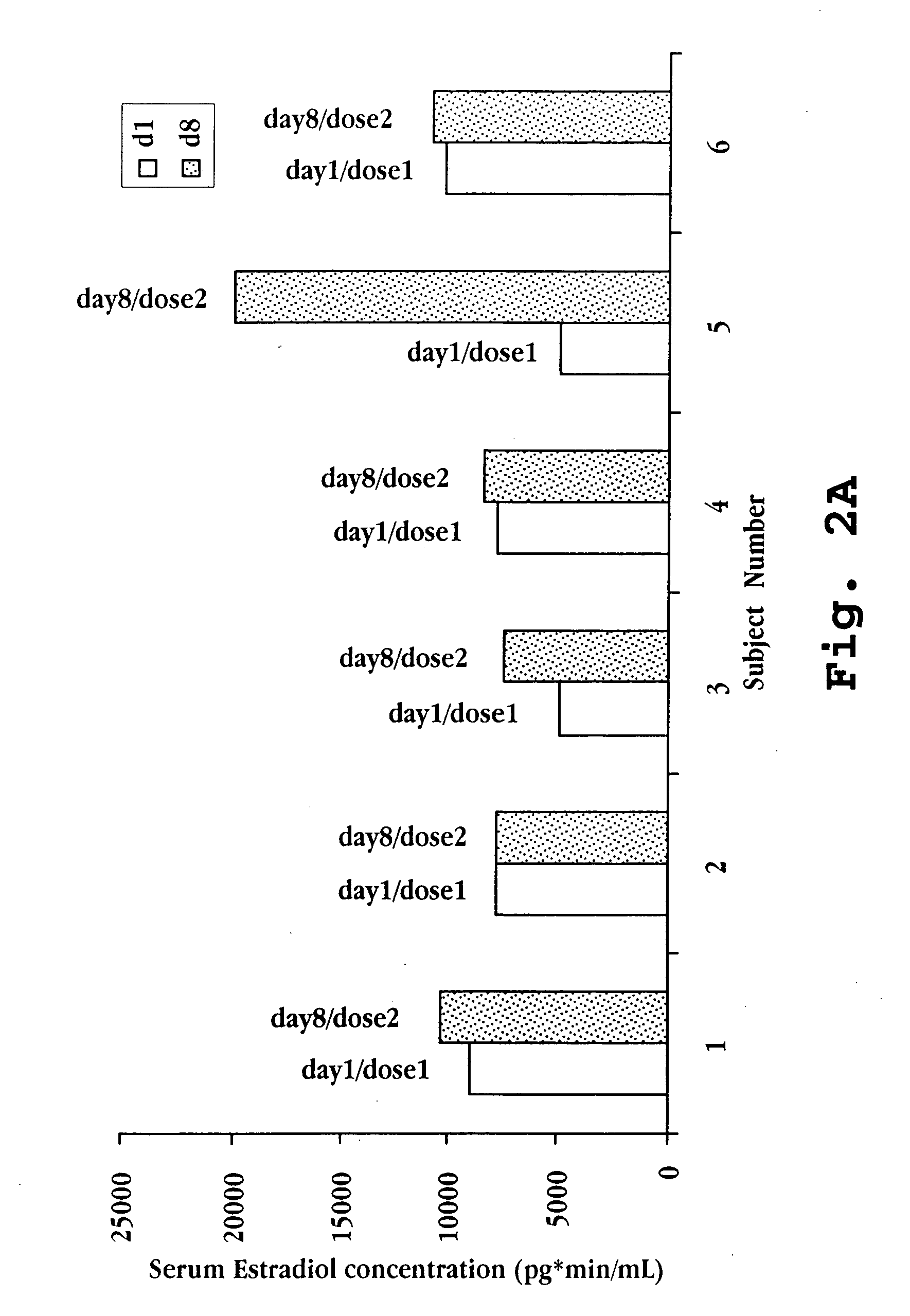
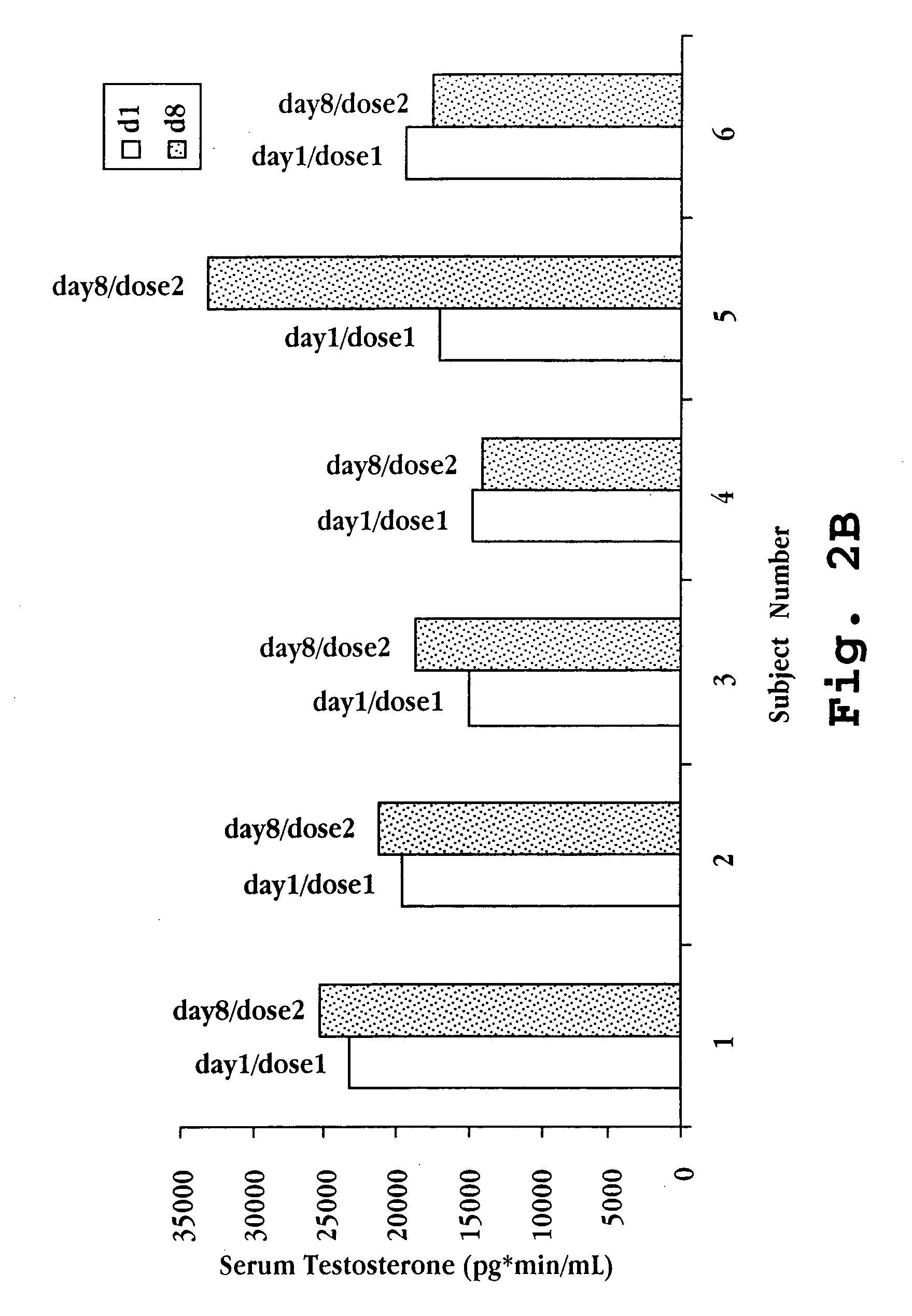
[0092] 2-Hydroxypropyl-β-cyclodextrin was added to water at a concentration of 240 mg / mL and stirred until dissolved. 17-β-Estradiol was then added to the water-cyclodextrin solution at a concentration of 1.0 mg / mL. The mixture was stirred until dissolution. Testosterone at a concentration of 5.0 mg / mL was then added, and after stirring to dissolution benzalkonium chloride (0.1 mg / mL), ethylene diamine tetra acetic acid (EDTA; 1 mg / mL), and sorbitol (61.6 mg / mL) were added. The mixture was stirred. Then, the GnRH compound deslorelin acetate was added at a concentration of 20 mg / mL with stirring. The volume was brought to the final desired volume and the pH was adjusted as needed. Table 4 summarizes the preparation components, concentrations, and dosages per 50 μL.
TABLE 4Components in Exemplary Nasal PreparationConcentrationComponent(mg / mL)Dose per 50 μLdeslorelin acetate201.0mg17-β-estradiol1.050μgTestosterone5.0250μg2-hydroxypropyl-β-cyclodex...
example 3
Intranasal Administration of Deslorelin to Premenopausal Women with Uterine Leiomyomata
[0093] A 12 week study was performed to establish an effective dose of deslorelin for controlling heavy bleeding secondary to uterine leiomyomata. Forty-one premenopausal women completed the study and are identified as Subject Nos. 1 through 41. The women were divided into test groups for treatment with intranasal deslorelin as follows:
Group 1Subject Nos. 1-6placebo, 0 mg deslorelinGroup 2Subject Nos. 7-210.5 mg deslorelin, once per dayGroup 3Subject Nos. 22-341.0 mg deslorelin, once per dayGroup 4Subject Nos. 35-412.0 mg deslorelin, once per day
The intranasal preparation consisted of deslorelin at the indicated concentration along with sorbitol (61.6 mg / mL), benzalkonium chloride (0.1 mg / mL), and water.
[0094] The average age of the women was 42.3 years, with similar distribution among groups. For one complete menstrual cycle prior to treatment, each woman completed a daily bleeding calendar....
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com