Identification and use of growth hormone secretagogue receptor type 1A antagonists
a technology of growth hormone secretagogue and receptor type, applied in the direction of biological testing, biological material analysis, dipeptide ingredients, etc., can solve the problems of reduced mobility, decreased quality of life, and no convincing treatment currently available for reducing body weight effectively and acceptably
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0061] Identification of Ligands for the Receptor GHS-R 1A
[0062] Transfection
[0063] Lipofectamine (Life Technologies, Rockville, Md., U.S.A.) was used for transfection of BHK cells with a GHS-R 1A expression vector (Howard, A. D. et al. (1996), Science 273, 974-977).
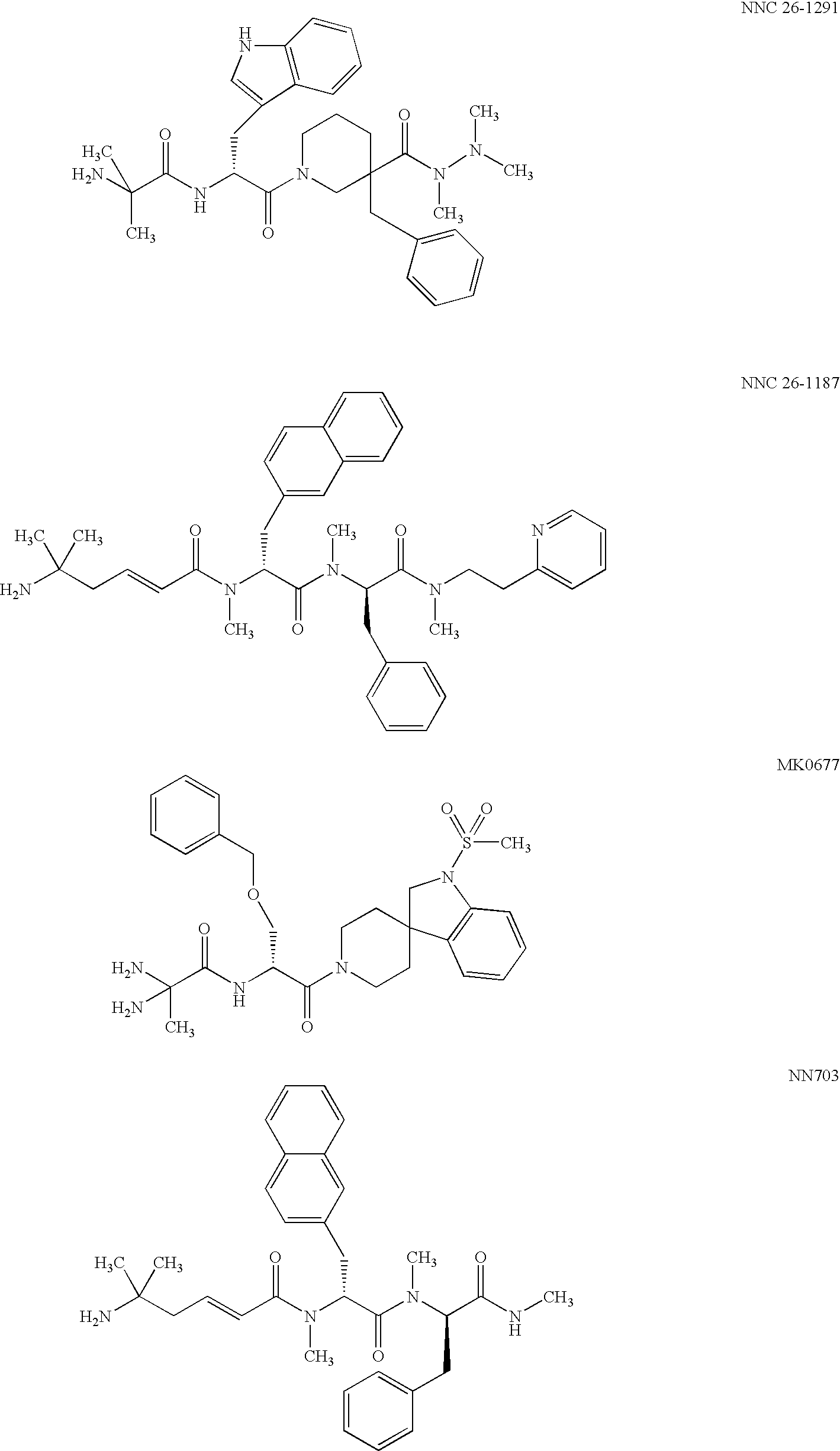
[0065] Receptor binding was assayed as described in Hansen, B. S. et al (1999) Eur. J. Endocrinol. 141:180-189. Briefly, crude membranes from stably transfected BHK / GHS-R 1A cells were suspended at 0.5 mg protein / ml in homogenization buffer (25 mM Tris-base, 2.5 mM EDTA, 10 mM MgCl2 and 30 μg / ml bacitracin). In a microtiter plate 10 μl membrane suspension was combined with either 35S-labelled MK0677 (see Example 4) (Amersham Pharmacia Biotech, Essex, UK) or 2-3H-adenosine (Amersham) as well as binding buffer (2.5 mM Tris-base, 2.5 mM EDTA and 10 mM MgCl2) to a total volume of 250 μl. Non-specific binding was determined by adding 10 μM MK0677 (see Example 4) or 10 μM adenosine to the assay...
example 2
[0071] Identification of Adenosine as a Ligand for the Receptor GHS-R 1A
[0072] The methods of example 1 were used. Adenosine was found to be a potent ligand for the GHS-R 1A (EC50˜50 nM using the Ca++ assay described above). Binding studies were performed to characterize the binding of adenosine to the GHS-R 1A, and a KD of 87±10 nM was determined.
[0073] Adenosine was, however, unable to stimulate GH secretion from rat pituitary cells (assay described e.g. in Hansen, B. S. et al (1999) Eur. J. Endocrinol. 141:180-189).
example 3
[0074] Adenosine Does Not Stimulate Growth Hormone Release, but Stimulates Feeding
[0075] The effect on GH release was studied in Halothane anaesthetized male Wistar rats after intracerebroventricular (icv) and intravenous (iv) administration of adenosine and also in pentobarbital anaesthetized catheterized female Sprague Dawley (SD) rats after iv administration. Vehicle or adenosine was given to groups of rats (n=4-6) in the following doses: 10 μg / rat and 100 μg / rat icv dissolved in 5 μ; saline, 1 mg / kg iv and also 10 mg / kg iv to the SD rats. Blood samples were obtained from anaesthetized animals before dosing, and either 10 min after dosing or by frequent blood sampling through a catheter up until 45 min after dosing. The plasma was analyzed for rat GH.
[0076] The effect on feeding was studied in conscious non-deprived male Wistar rats (n=7-10) after icv dosing of vehicle or adenosine (1 μg / rat and 10 μg / rat in 5 μl saline). Food intake was measured in feeding boxes with standard ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Current | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com