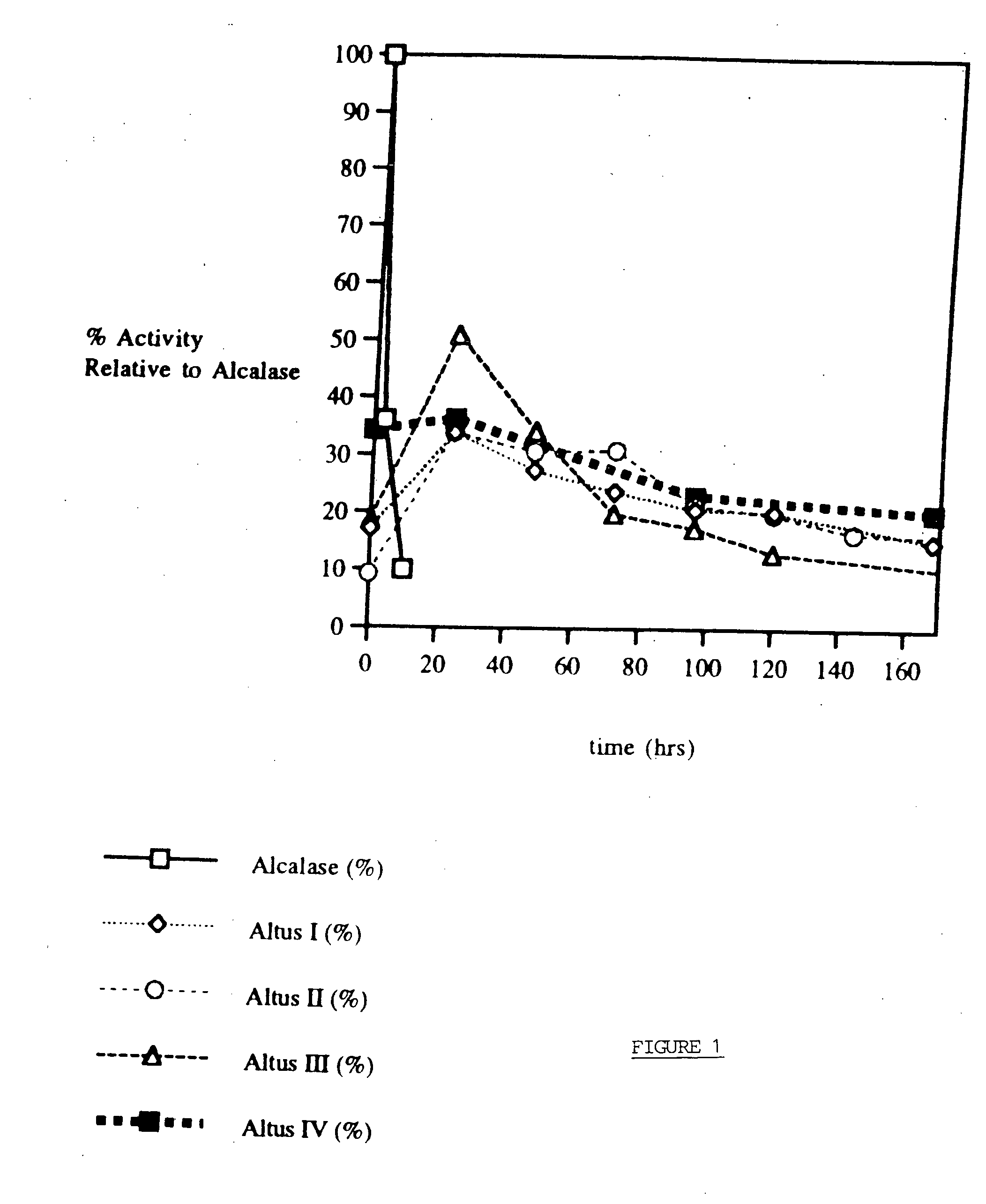
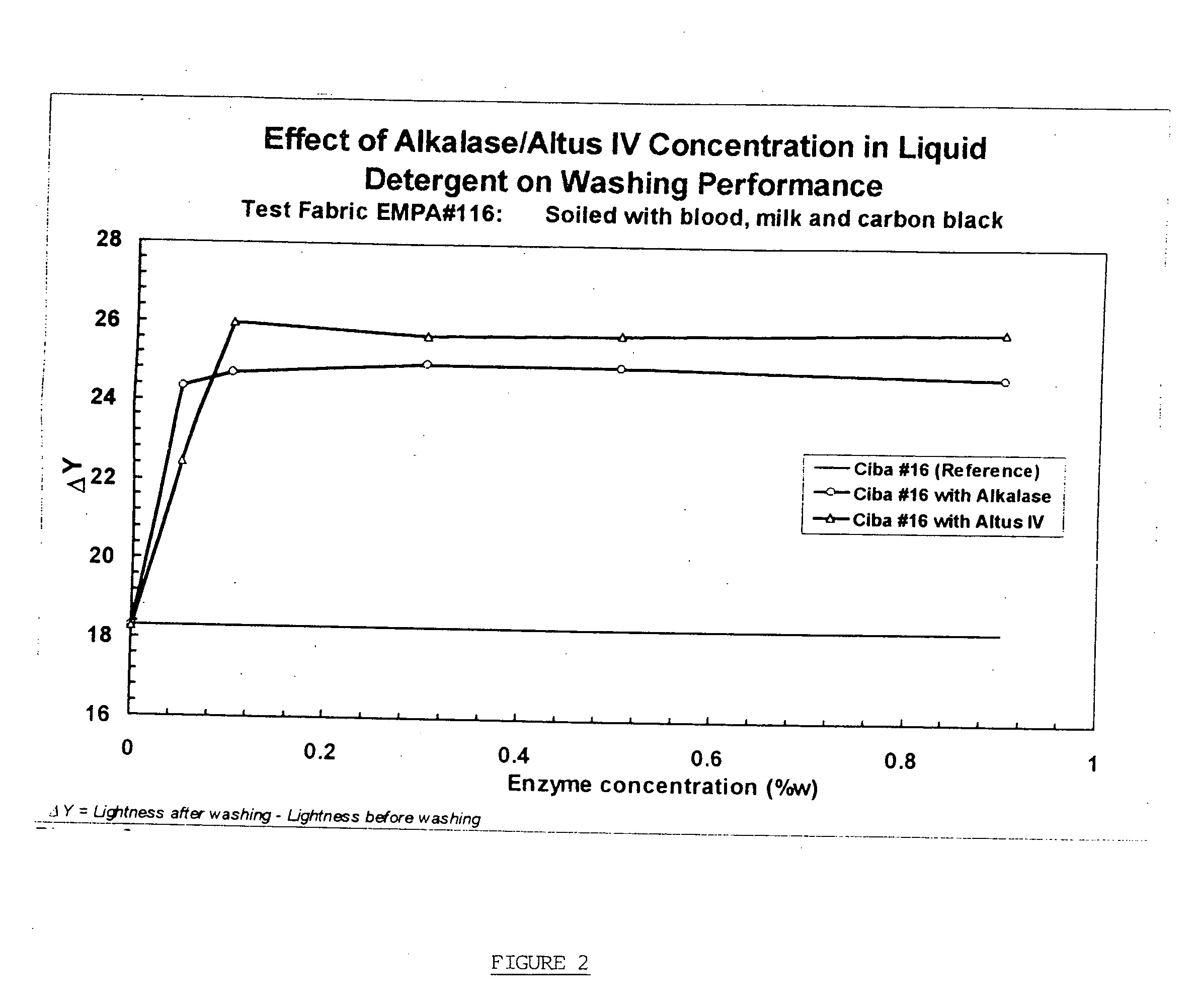
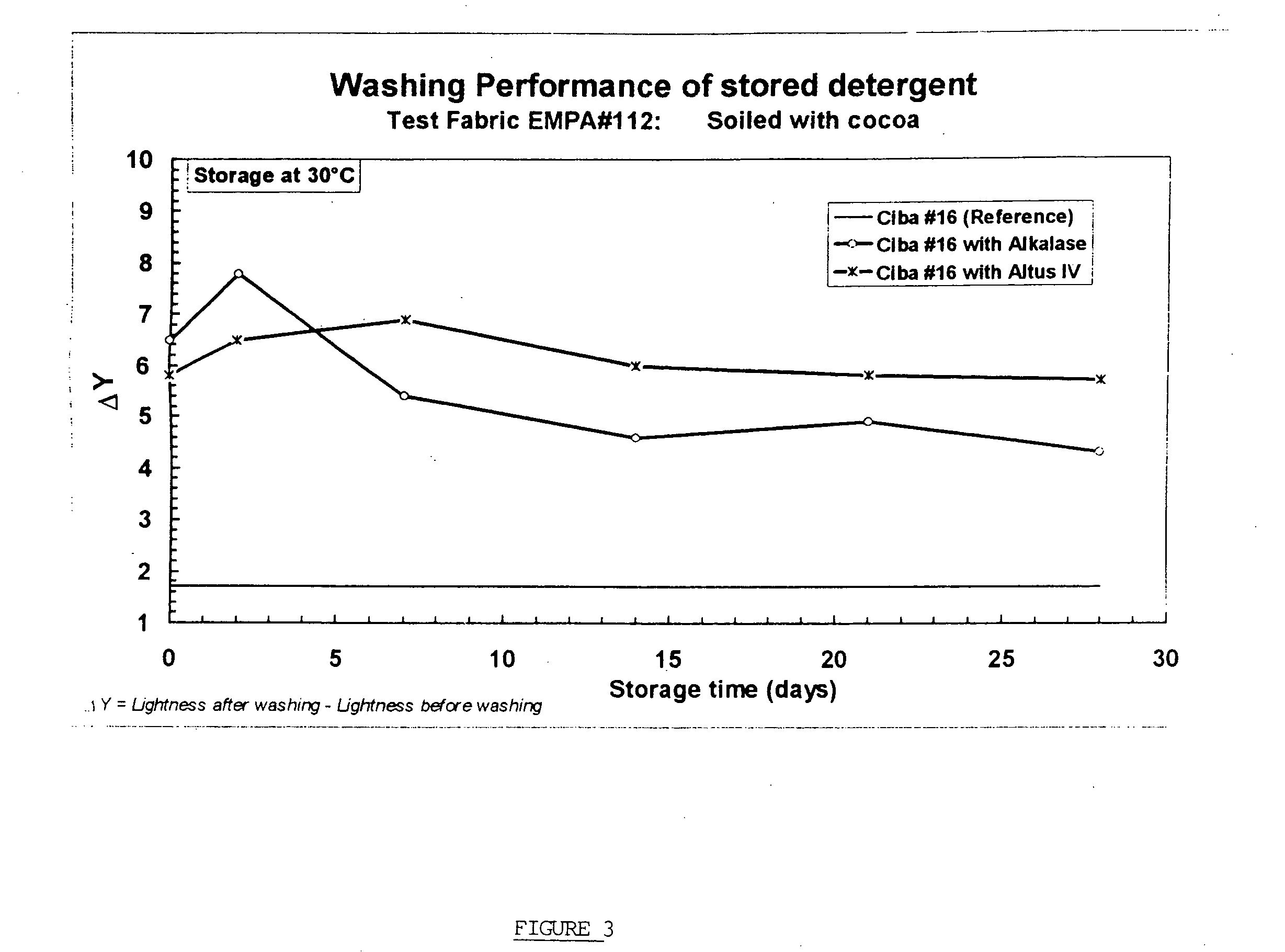
Controlled dissolution crosslinked protein crystals
a crosslinked protein and crystal technology, applied in the direction of oxidoreductases, enzyme stabilisation, immobilised enzymes, etc., can solve the problems of poor stability of proteins, variability of performance or high cost, and limited us
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Crosslinked Subtilisin Crystals
[0115] One volume of Alcalase 2.5 L (Novo Nordisk Bioindustrials, Franklinton, N.C.) was added to 2 volumes of a solution of 15% sodium sulfate (pH 5.5) prepared at 30-35° C. The crystallization solution was seeded with 1 / 2,000-1 / 500 volume seeds (30 mg / ml slurry of crystals in 15% sodium sulfate (pH 5.5), pH supported at 5.5 by adding NaOH. The seeded crystallization solution was incubated at 30-35° C., stirring by magnetic stirrer overnight. This yielded 60-80% (by activity) crystal rods, 10-50 μm, in length, 1-3 μm in width, after 24-48 hours.
example 2
Crosslinking of Subtilisin Crystals
[0116] Subtilisin crystals were crosslinked using one of a variety of crosslinkers, including: glutaraldehyde, glyoxal, succinaldehyde, octanedialdehyde and epoxides.
Glutaraldehyde Crosslinking
[0117] Glutaraldehyde (“GA”) (supplied as 50% in aqueous by Aldrich Chemical Co.) was diluted in deionized water at 4° C. in the various amounts listed in Table I below. For each ml of subtilisin crystals (27 mg / ml) in 15% sodium sulfate, 10 μl of the diluted glutaraldehyde was added to the slurry while shaking on a vortex at low speed (for amounts less than 5 ml) or stirring with an overhead stirrer at medium speed (for amounts 25 ml-500 ml). After mixing for the allotted crosslinking time, the samples were centrifuged for 20 seconds at maximum speed, the supernatant was discarded and replaced with 15% sodium sulfate. This “washing” was repeated a total of 5 times. The final resuspension was effected with 900 μl of 15% sodium sulfate.
TABLE IGlutaraldeh...
example 3
Activity Assay
[0136] In order to test the activity of crosslinked protein crystals according to this invention, as well as other enzyme samples, we developed the following azocasein assay.
[0137] Materials:
[0138] 2.0M Tris Buffer. 500 ppm CaCl2
[0139] 0.2M Tris Buffer. 50 ppm CaCl2
[0140] 50% urea
[0141] Azocasein
[0142] 5% trichloroacetic acid (“TCA”)
[0143] Alcalase (2.5 L)
[0144] ChiroCLEC-BL™ (crosslinked subtilisin crystals, available from Altus Biologics, Inc., Cambridge, Mass.)
[0145] The assay was carried out, preparing azocasein just prior to use, by dissolving 600 mg azocasein with 10 ml of 50% urea and vortexing lightly to complete the dissolution. Then 10 ml 2.0M Tris was added and vortexed to mix, increasing the volume to 100 ml by adding deionized water.
[0146] The stock solutions of the enzyme to be assayed in 0.2M Tris were prepared, to provide 50 μl aliquots to be assayed, as follows:
Without detergent: 0.03 mg / ml Alcalase (soluble, uncrosslinked subtilisin Carls...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com