Biomarkers and methods for determining sensitivity to microtubule-stabilizing agents
a microtubule stabilizing agent and biomarker technology, applied in the field of pharmaceuticals, can solve problems such as the difficulty of predicting drug sensitivity in patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Identification of Biomarkers
Methods
[0068] Cell Lines and Cytotoxicity Assay
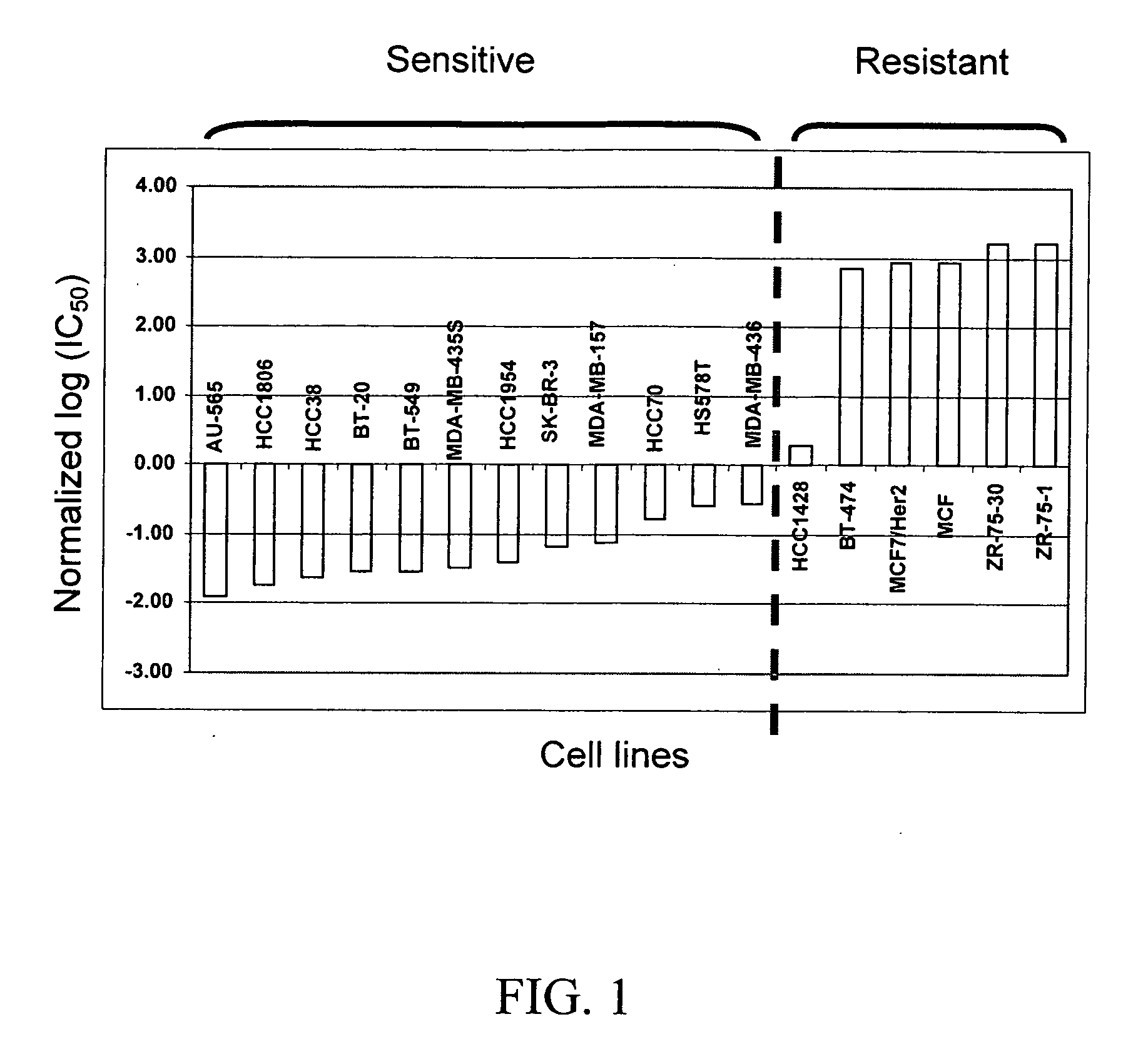
[0069] 23 breast cancer cell lines were assayed for their sensitivity to ixabepilone. Each cell line was exposed to ixabepilone for 72 hours, and growth inhibition was assessed by the CellTiter 96® Aqueous Non-Radioactive Cell proliferation Assay (Promega) for IC50 measurements. Then, the concentration of the ixabepilone required for 50% growth inhibition was calculated as the IC50. For each experimental condition, at least triplicate measurements were carried out for each cell line. The 23 cell lines were assayed for their IC50 measurements twice, and these two separate IC50 data sets were used for the following analysis.
[0070] Training Set Selection
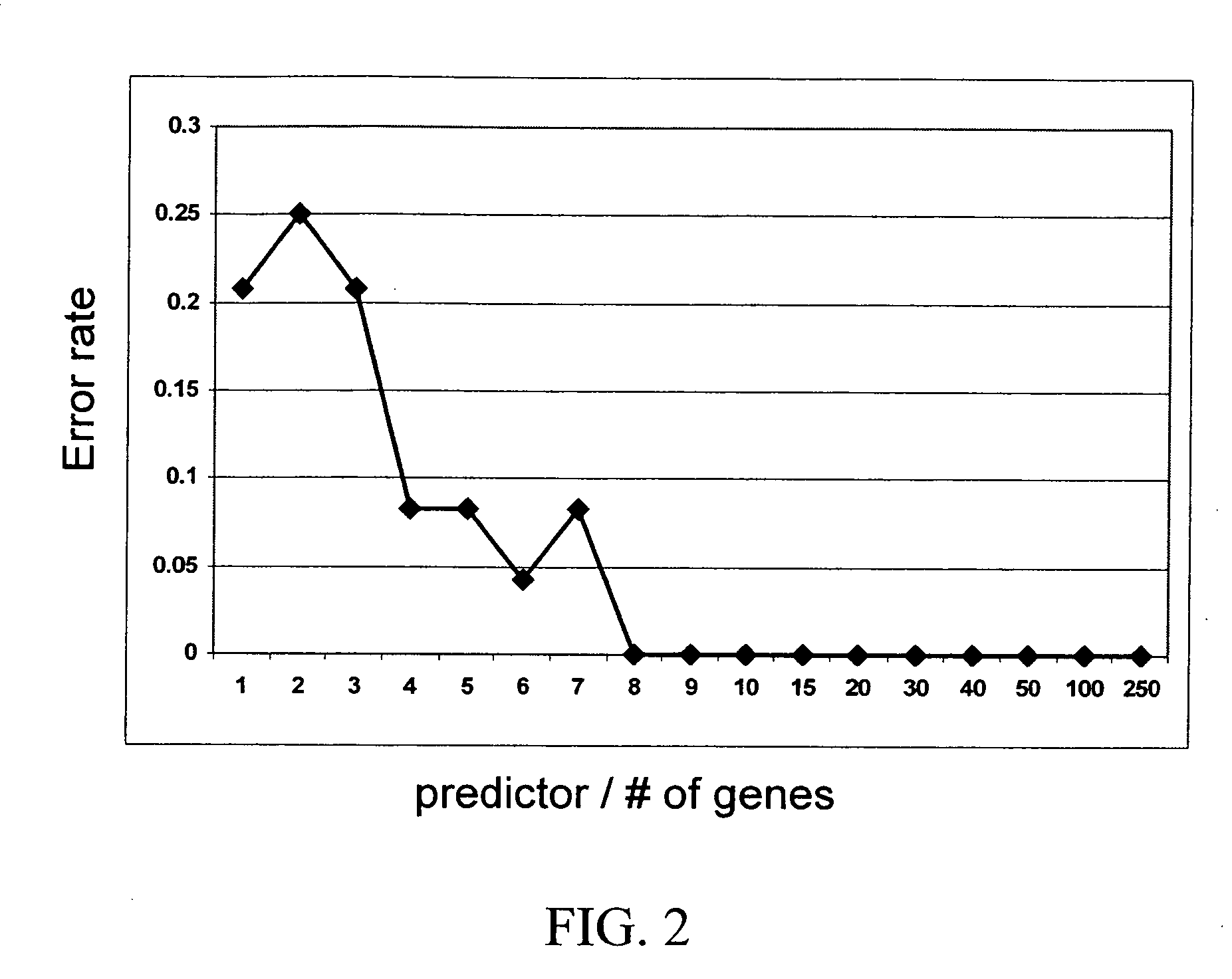
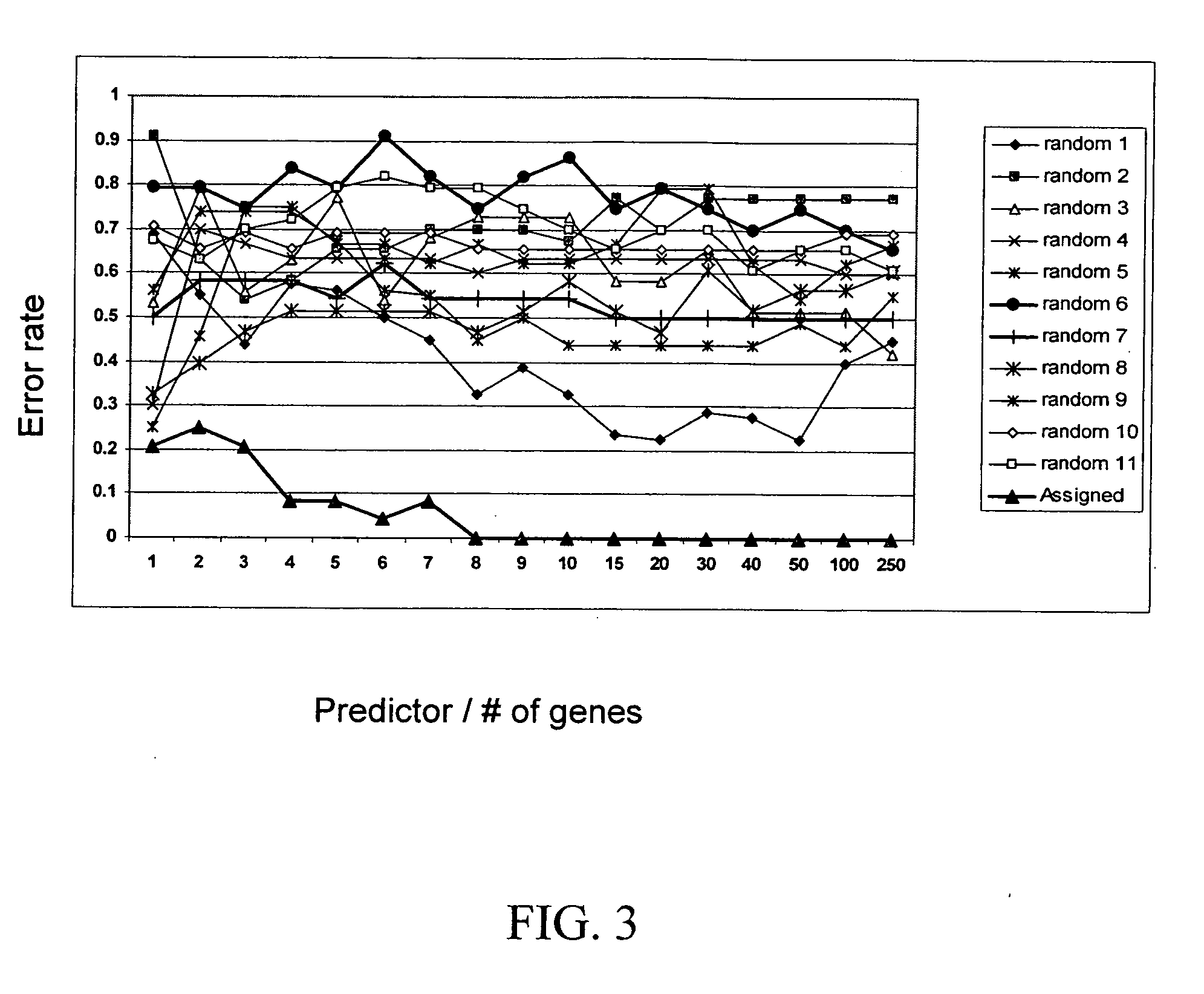
[0071] For analysis, training cell lines were chosen in the following manner. The 23 cell lines were assigned into the classes “sensitive” or “resistant” using IC50 values; log(IC50) values were normalized based on the mean and the standard deviation (SD) a...
example 2
Further Evaluation of ER and Tau Biomarkers
[0091] Estrogen receptor (ER) and tau (Tau) were identified as biomarkers since their expression patterns were highly correlated with resistance to ixabepilone. In addition, it was found that the ER pathway was the most implicated biological network for resistance to ixabepilone based on the pathway analysis using preclinical candidate markers (FIGS. 9 and 10). Interestingly, Tau was recently identified as the gene most correlated with pathological complete response for T / FAC neoadjuvant treatment in breast cancer patients (M. Ayers et al, J. Clin. Oncol., 22(12):2284-93 (2004); R. Rouzier et al., P.N.A.S., Jun 7; 102(23):8315-20 (2005)). Following this report, our preclinical study on paclitaxel supported the clinical findings of Tau as a novel mediator of paclitaxel sensitivity (P. Wagner et al., Cell Cycle, Sep; 4(9):1149-52 (2005)). ER and Tau were evaluated for their predictability of response to ixabepilone in CA163-080 trial.
Metho...
example 3
Production of Antibodies Against the Biomarkers
[0105] Antibodies against the biomarkers can be prepared by a variety of methods. For example, cells expressing a biomarker polypeptide can be administered to an animal to induce the production of sera containing polyclonal antibodies directed to the expressed polypeptides. In one aspect, the biomarker protein is prepared and isolated or otherwise purified to render it substantially free of natural contaminants, using techniques commonly practiced in the art. Such a preparation is then introduced into an animal in order to produce polyclonal antisera of greater specific activity for the expressed and isolated polypeptide.
[0106] In one aspect, the antibodies of the invention are monoclonal antibodies (or protein binding fragments thereof). Cells expressing the biomarker polypeptide can be cultured in any suitable tissue culture medium, however, it is preferable to culture cells in Earle's modified Eagle's medium supplemented to contain...
PUM
| Property | Measurement | Unit |
|---|---|---|
| total volume | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| multidrug resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com