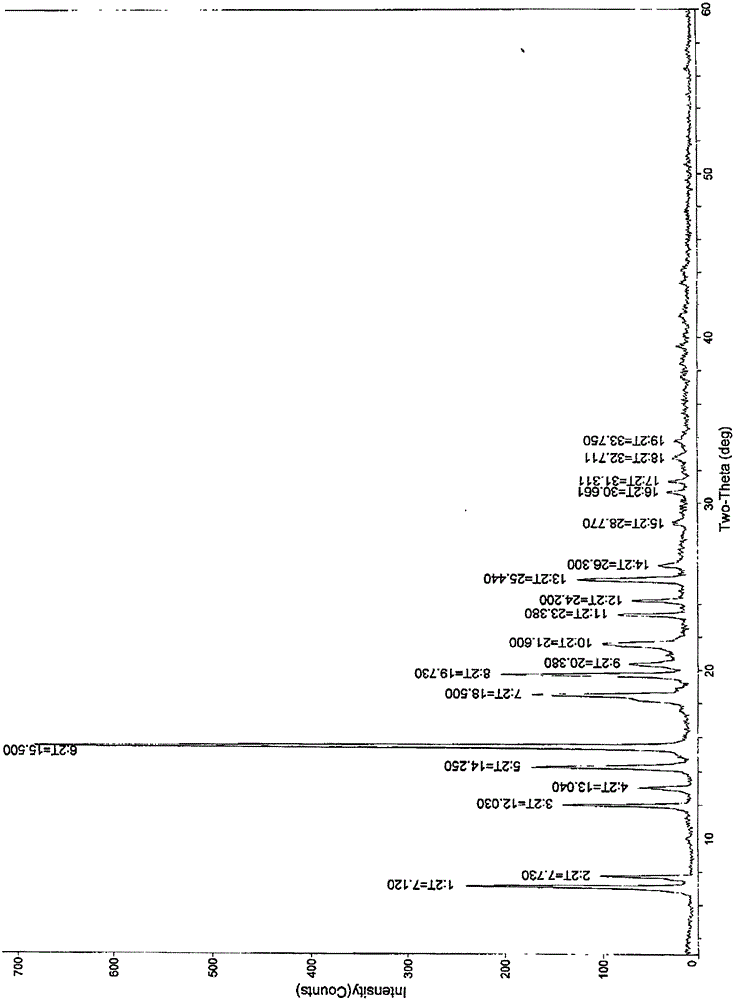
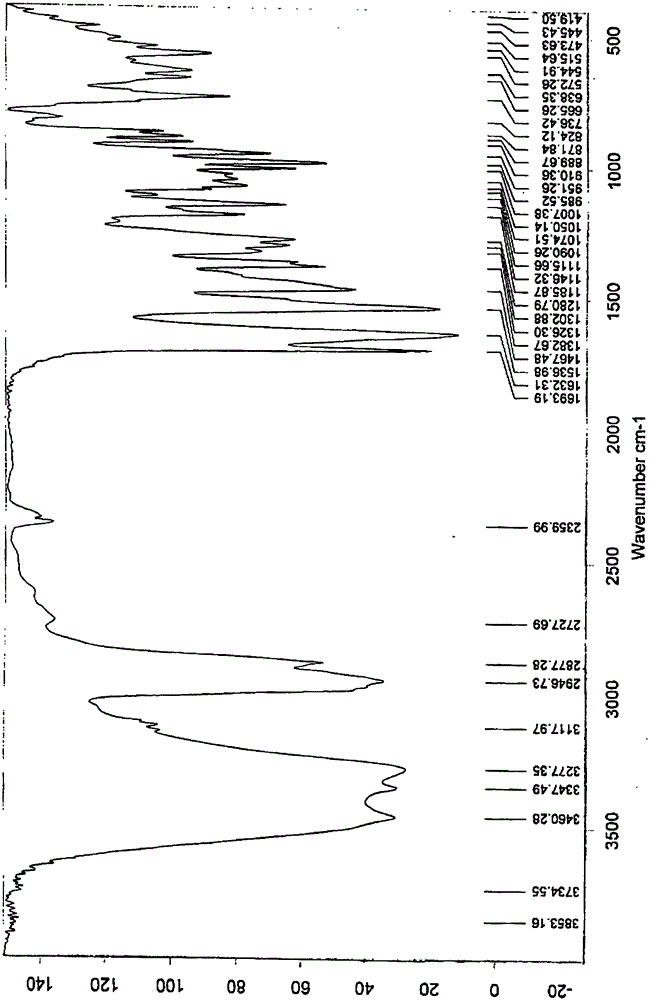
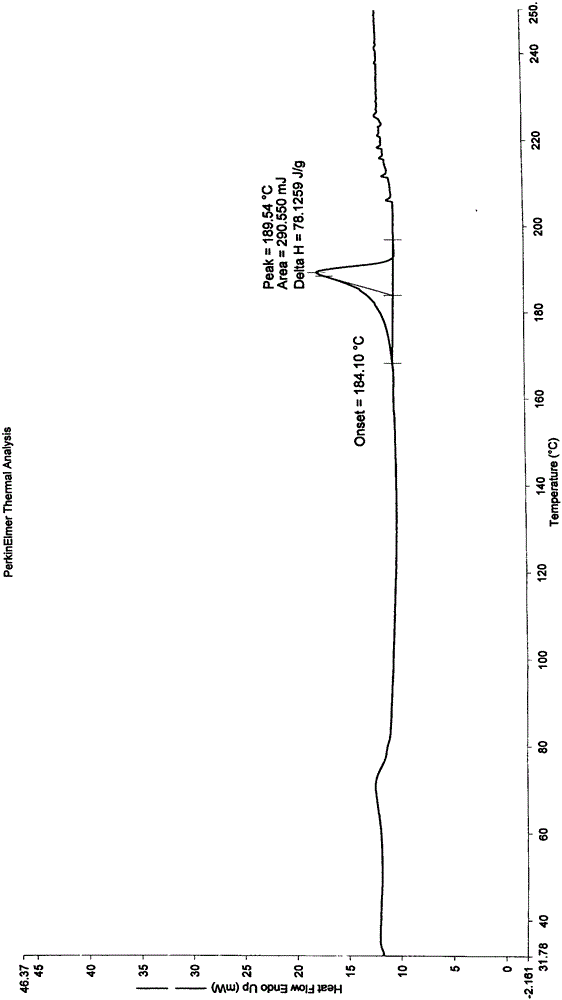
New crystal form of ixabepilone and preparation method thereof
A technology of ixabepilone and crystal form, applied in the field of pharmaceuticals, can solve the problem of low susceptibility of tumor drug resistance mechanism
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Dissolve 0.4g of ixabepilone crude product (HPLC purity 99%) in 10ml of tetrahydrofuran, filter, and place the filtrate in a ventilated place away from light, and wait for tetrahydrofuran to volatilize and precipitate asbestos-like solids, filter, and dry in vacuo to obtain ixabepilone Form M.
Embodiment 2
[0048] Ixabepilone crude product 0.4g (HPLC purity 99%) was dissolved in 20ml of tetrahydrofuran, filtered, and the filtrate was placed in a ventilated place to avoid light, and the asbestos-like solid was precipitated after tetrahydrofuran volatilized, filtered, and vacuum-dried to obtain Ixabepilone Form M.
Embodiment 3
[0050] Ixabepilone crude product 0.4g (HPLC purity 99%) was dissolved in 30ml of tetrahydrofuran, filtered, and the filtrate was placed in a ventilated place to avoid light, and the asbestos-like solid was precipitated after tetrahydrofuran volatilized, filtered, and vacuum-dried to obtain Ixabepilone Form M.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com