A freeze-dried composition of ixabepilone albumin and its preparation method
A technology of albumin and composition, which is applied in the direction of drug combination, freeze-dried delivery, non-active ingredients of polymer compounds, etc. Problems such as poor reproducibility, achieve good redispersibility, good uniformity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
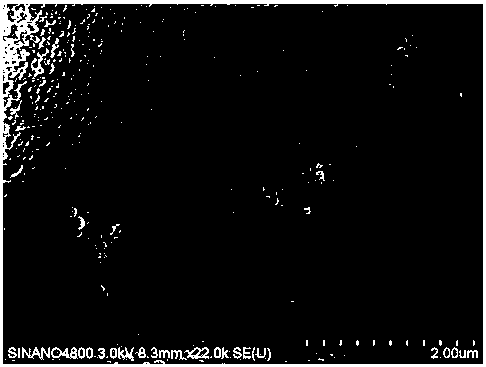
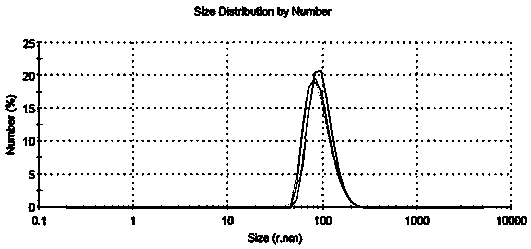
[0041] Weigh 0.20g of Ixabepilone crude drug and dissolve it in 20.0ml of a mixed solvent of absolute ethanol and chloroform (1:5) to obtain a solution with a concentration of 10mg / mL, and pour it into the storage tank of the dispersed phase container; Then weigh 2.0 g of human serum albumin and dissolve it in 100 ml of water for injection (the pH is adjusted to 5.5 by citric acid) as the continuous phase, add the continuous phase to the continuous phase vessel, turn on the plunger pump to circulate the continuous phase, and keep the continuous phase flow rate at 20mL / min, open the valve to make the dispersed phase slowly pass through the membrane (pore size 0.1um) under a constant pressure of 50KPa and press it into the continuous phase until the pressure indicator drops, collect, centrifuge the resulting suspension, collect the precipitate, and pre-freeze to- The ixabepilone freeze-dried composition is obtained by freeze-drying at a temperature of below 40°C for 24 hours, whil...
Embodiment 2
[0043] Weigh 0.20g of Ixabepilone crude drug and dissolve it in 20.0ml of a mixed solvent of absolute ethanol and chloroform (1:5) to obtain a solution with a concentration of 10mg / mL, and pour it into the storage tank of the dispersed phase container; Then weigh 2.0 g of human serum albumin and dissolve it in 100 ml of water for injection (the pH is adjusted to 5.5 by citric acid) as the continuous phase, add the continuous phase to the continuous phase vessel, turn on the plunger pump to circulate the continuous phase, and keep the continuous phase flow rate at 50mL / min, open the valve to make the dispersed phase slowly pass through the membrane (pore size 0.1um) under a constant pressure of 200KPa and press into the continuous phase until the pressure indicator drops, collect, centrifuge the resulting suspension, collect the precipitate, and pre-frozen until- The ixabepilone freeze-dried composition is obtained by freeze-drying at a temperature of below 40°C for 24 hours, whi...
Embodiment 3
[0045] Weigh 0.20g of Ixabepilone crude drug and dissolve it in 20.0ml of a mixed solvent of absolute ethanol and chloroform (1:5) to obtain a solution with a concentration of 10mg / mL, and pour it into the storage tank of the dispersed phase container; Then weigh 2.0 g of human serum albumin and dissolve it in 100 ml of water for injection (the pH is adjusted to 5.5 by citric acid) as the continuous phase, add the continuous phase to the continuous phase vessel, turn on the plunger pump to circulate the continuous phase, and keep the continuous phase flow rate at 50mL / min, open the valve to make the dispersed phase slowly pass through the membrane (pore size 0.4um) under a constant pressure of 200KPa and press into the continuous phase until the pressure indicator drops, collect, centrifuge the resulting suspension, collect the precipitate, and pre-freeze to- The ixabepilone freeze-dried composition is obtained by freeze-drying at a temperature of below 40°C for 24 hours, while ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com