Stable ixabepilone pharmaceutical composition
A technology of ixabepilone and composition, applied in the field of ixabepilone pharmaceutical composition and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
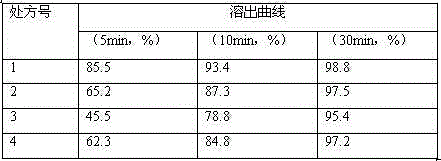
Examples
Embodiment 1
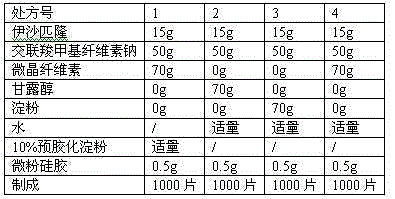
[0033] The formulation of ixabepilone made into 1000 tablets is as follows:
[0034] Ixabepilone 15g
[0035] Croscarmellose Sodium 50g
[0036] Microcrystalline cellulose 70g
[0037] Micropowder silica gel 0.5g
[0038] Appropriate amount of 10% pregelatinized starch solution
[0039] Preparation
[0040] 1) Ixabepilone, croscarmellose sodium and microcrystalline cellulose were dried, pulverized, and passed through a 100-mesh sieve to obtain ixabepilone powder, croscarmellose sodium and microcrystalline cellulose respectively. Cellulose powder;
[0041] 2) Take the prescribed amount of ixabepilone, croscarmellose sodium and microcrystalline cellulose, mix well, then add 10% pregelatinized starch solution to make a soft material, pass through a 40-mesh sieve to granulate , to obtain ixabepilone wet granules;
[0042] 3) Dry the above-mentioned ixabepilone wet granules at 60°C until the water content is less than 5%, to obtain ixabepilone dry granules
[0043] 4) Pass ...
Embodiment 2
[0045] The formulation of ixabepilone made into 1000 tablets is as follows:
[0046] Ixabepilone 45g
[0047] Croscarmellose Sodium 100g
[0048] Microcrystalline cellulose 140g
[0049] Micropowder silica gel 1g
[0050] Appropriate amount of 10% pregelatinized starch solution
[0051] Preparation
[0052] 1) Ixabepilone, croscarmellose sodium and microcrystalline cellulose were dried, pulverized, and passed through a 100-mesh sieve to obtain ixabepilone powder and microcrystalline cellulose powder respectively;
[0053] 2) Take the prescribed amount of ixabepilone, croscarmellose sodium and microcrystalline cellulose, mix well, then add 10% pregelatinized starch solution to make a soft material, pass through a 40-mesh sieve to granulate , to obtain ixabepilone wet granules;
[0054] 3) drying the above-mentioned ixabepilone wet granules at 60°C until the water content is less than 5%, to obtain ixabepilone dry granules;
[0055] 4) Pass the above-mentioned dry ixabepi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com