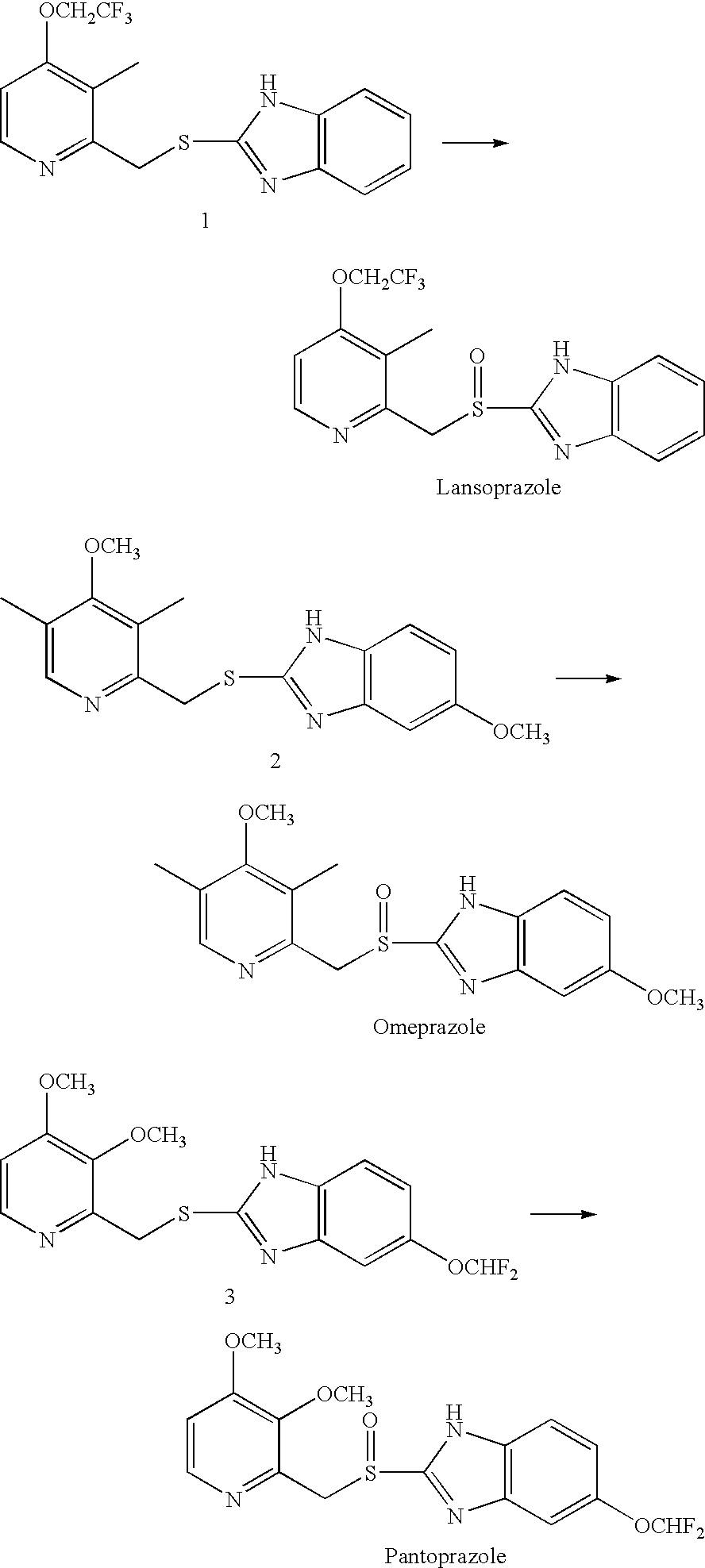
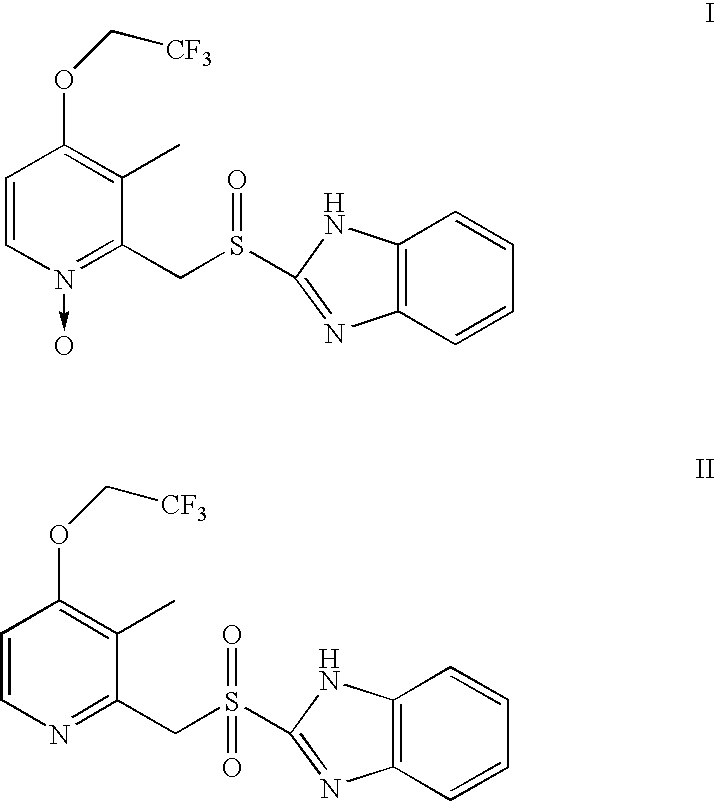
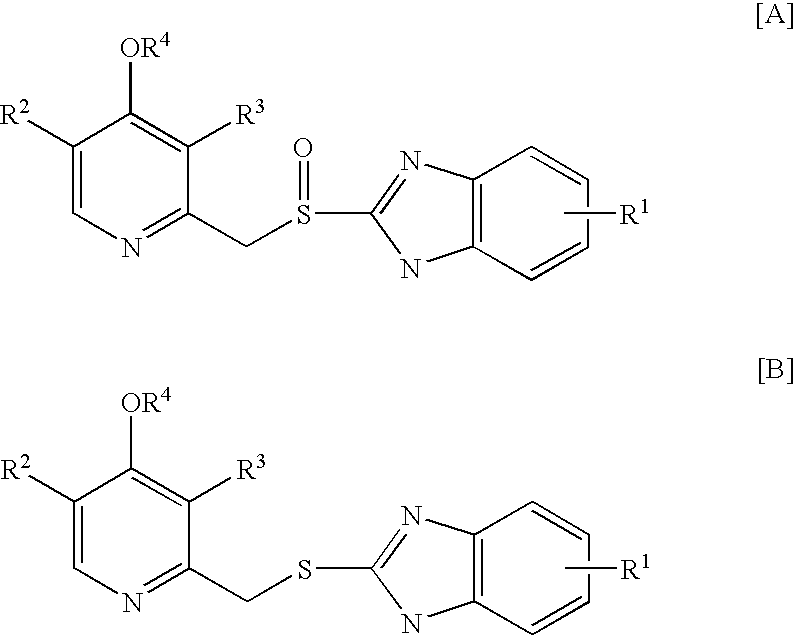
Method for preparing 2- (2-pyridylmethylsulphinyl) benzimidazoles
a technology of benzimidazoles and pyridylmethylsulphinyl, which is applied in the field of preparation of anti-ulcer agents, can solve the problems of poor quality control of methods, substantial mass production difficulties, and high cost of mcpba, and achieve mild reaction conditions and less toxic effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0020] 2.68 g of 2-chloromethyl-3-methyl-4-(2,2,2-trifluoroethoxy)pyridine.HCl, 1.376 g of 2-mercaptobenzimidazole, and 0.134 g of benzyl triethyl ammonium chloride as an interphase transfer catalyst were mixed in 24 ml of dichloromethane. 0.9534 g of NaOH (40%) / 12 ml water mixture solution was dripped into the above mixture while stirring. The temperature of the resulting solution was raised to 40° C. for about 2 hours. Then, dichloromethane was removed from the mixture under a reduced pressure. The solid obtained was stirred with 50 ml of water, and filtered to obtain 3.28 g of solid Lansoprazole precursor: 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methylthio]-1H-benzimidazole. At room temperature, 3.28 g of the above precursor, 0.1625 g of polyethylene glycol-400 as an interphase transfer catalyst, and 0.3936 g of Mo(acac)2 as an oxidation catalyst were mixed in 45 ml of isopropanol (abbreviated as IPA). To the resulting mixture 3.06 g of 35% H2O2 aqueous solution was adde...
example 2
[0021] At room temperature, 1.307 g of 2-[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl]methylthio]-1H-benzimidazole, 0.059 g of tetrabutyl ammonium bromide as an interphase transfer catalyst, and 0.157 g of Mo(acac)2 oxidation catalyst were mixed in 15 ml of IPA. Next, 1.36 g of TBHP (70% aqueous solution) was added into the mixture in about 5-10 minutes. The reaction was carried out for about 30 hours, and then 60 ml of water was added, and the reaction was continued for another one hour while stirring. Finally, the precipitate formed was filtered, water washed, and dried to obtain Lansoprazole with a yield of about 37% (HPLC purity >96%).
example 3
[0022] 3 g of Omeprazole precursor: 2-[(3,5-dimethyl-4-methoxy-2-pyridyl)methylthio]-5-methoxy-1H-benzimidazole, 0.09 g of Mo(acac)2 oxidation catalyst were dissolved in 20 ml of methanol by stirring. The temperature of the resulting solution was reduced to 0-5° C., followed by adding 1.17 g of 35% H2O2 aqueous solution. The reaction was carried out for about two hours, and then 60 ml of water was added, and the reaction was continued for another one hour while stirring. Finally, the precipitate formed was filtered, water washed, and dried to obtain Omeprazole with a yield of about 91-92% (HPLC purity >98%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com