Medicaments for the Treatment or Prevention of Fibrotic Diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example b1
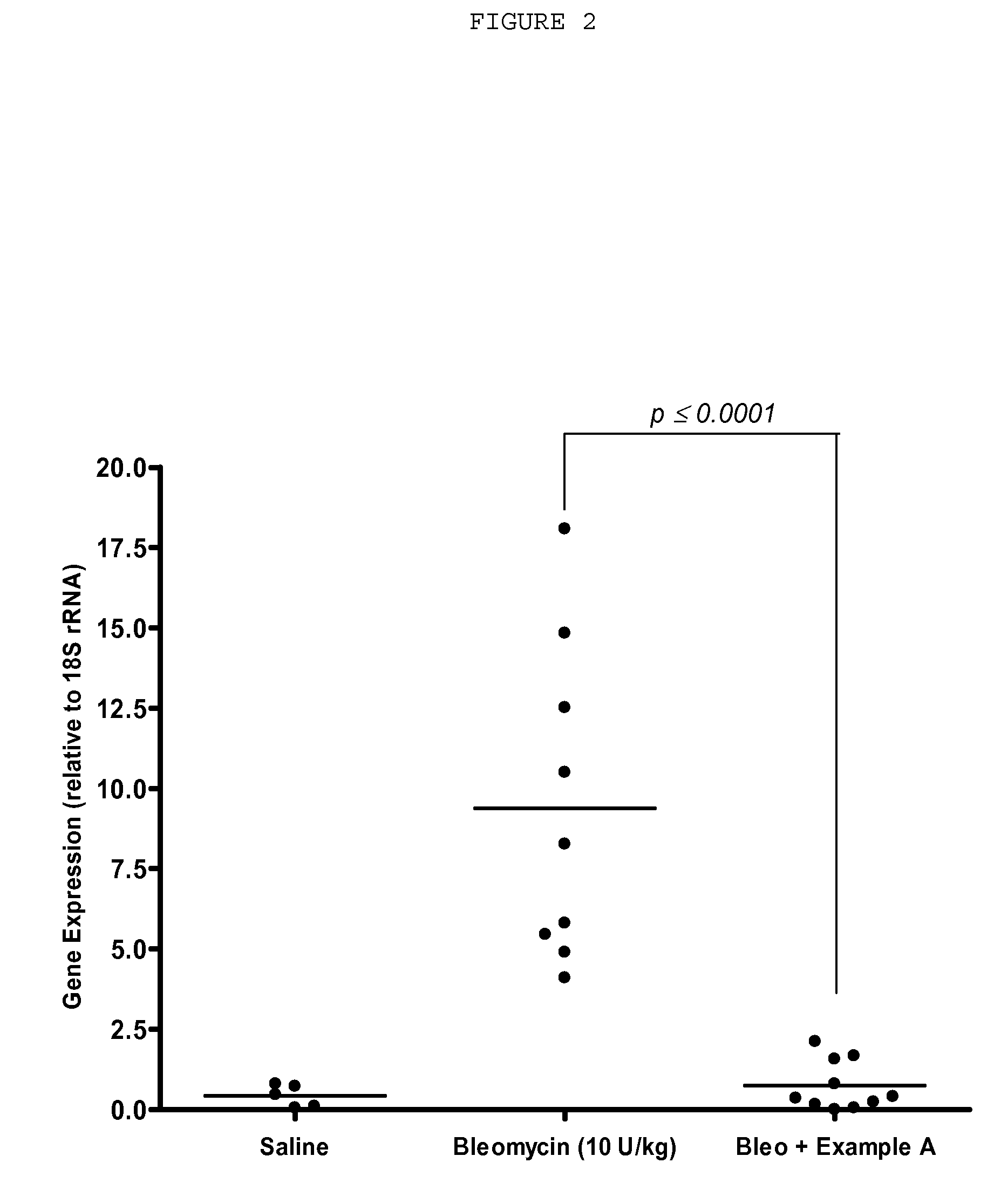
[1423] In the following experiments of Example B1, Example A denotes the compound 3-Z-[1-(4-(N-dimethylaminomethylcarbonyl-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone, which is compound (m) of the list of the preferred compounds.
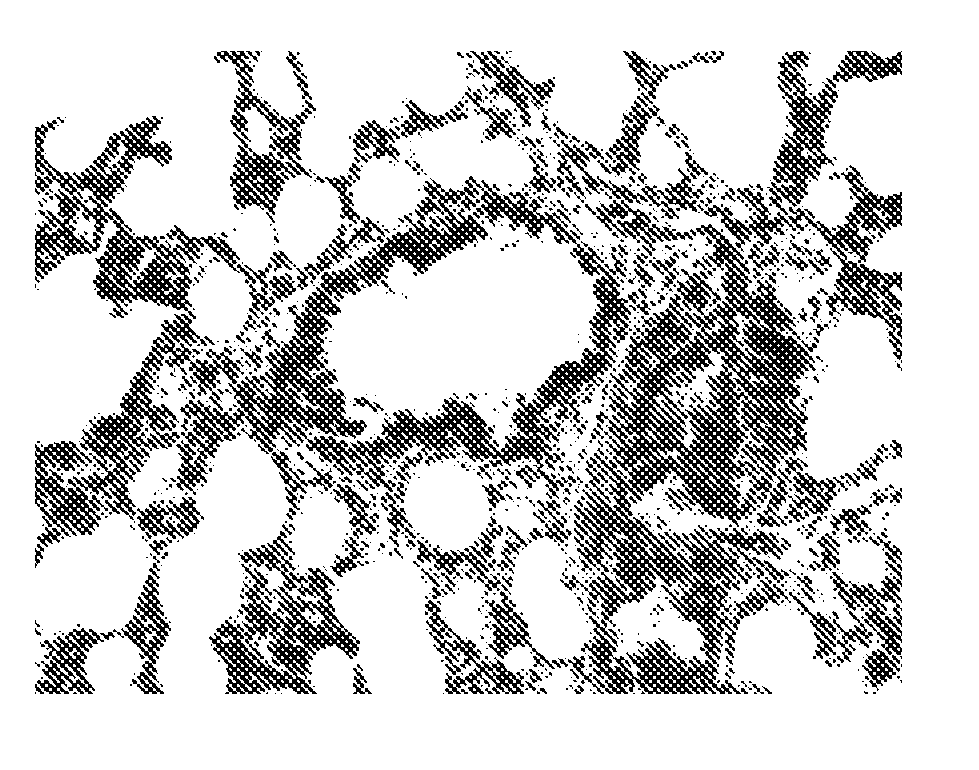
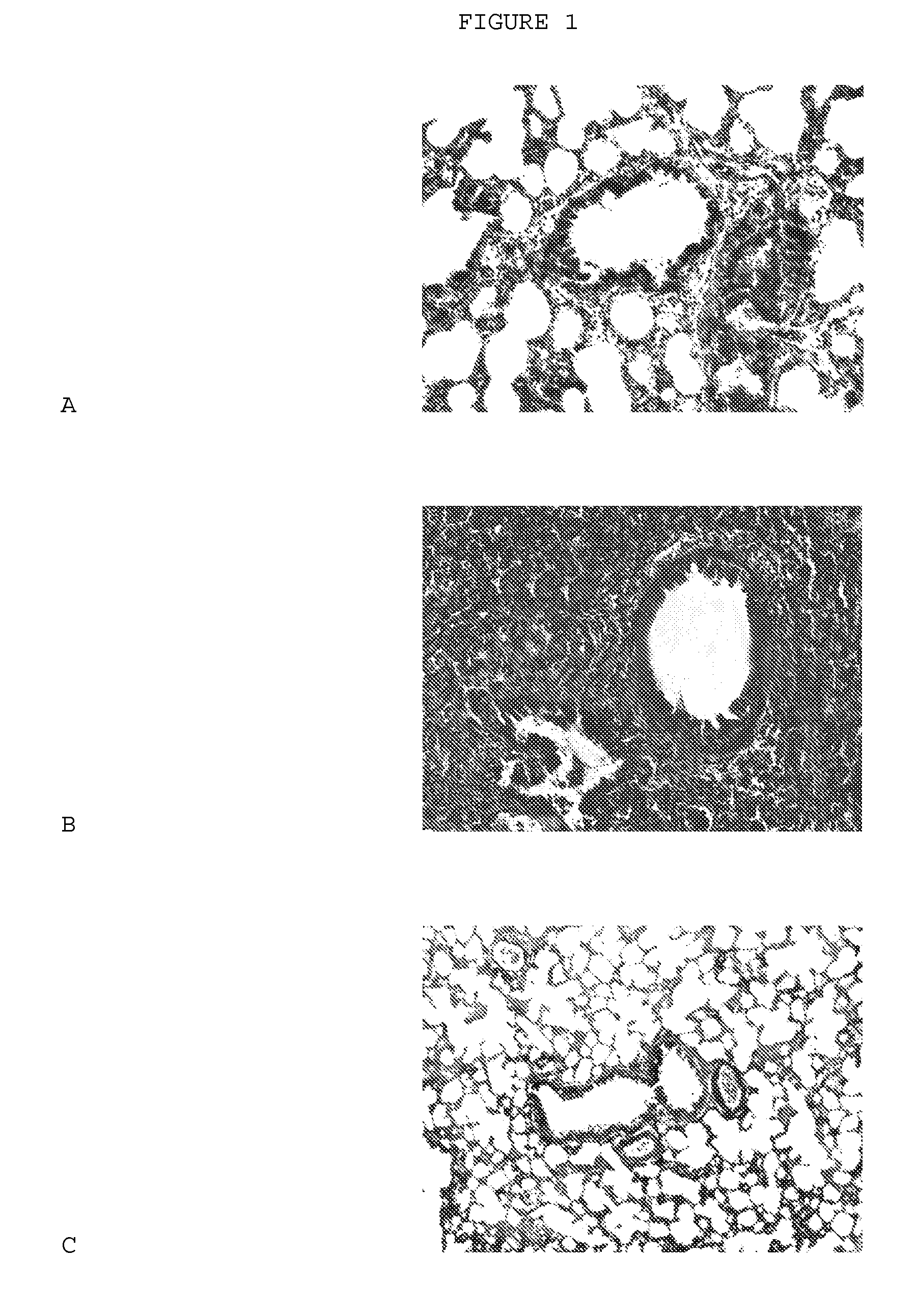
[1424] (A) Effect of a representative compound on lung morphology following bleomycin-induced pulmonary fibrosis.
[1425] Materials and Methods
[1426] Bleomycin sulfate (Bleomycin HEXAL™) was purchased from a local pharmacy.
[1427] Bleomycin Administration and Treatment Protocols
[1428] All experiments were performed in accordance with German guidelines for animal welfare, performed by persons certified to work with animals and approved by the responsible authorities. Male Wistar rats were intratracheally injected with Bleomycin sulfate (10 U / kg body weight in 300 μl saline) or saline alone (saline control) using a catheter (0.5 mm internal diameter, 1.0 mm external diameter) through the nasal passage, following exposure to th...
example b2
[1452] In the following experiments of Example B2, the compound 3-Z-[1-(4-(N-((4-methyl-piperazin-1-yl)-methylcarbonyl)-N-methyl-amino)-anilino)-1-phenyl-methylene]-6-methoxycarbonyl-2-indolinone, which is compound (u) of the list of the preferred compounds, was used.
[1453] All the methods employed are the same as the methods described in the experiments of Example B1, however using compound (u) instead of compound (m).
[1454] (A) Effect of a representative compound on lung morphology following bleomycin-induced pulmonary fibrosis.
[1455] Samples were prepared from rats treated as outlined in above Table 1 of experimental Example B1 (A).
[1456] Results
[1457]FIG. 4A shows the result obtained with the control group, which received saline and the vehicle instead of bleomycin intratracheally.
[1458] Rats treated intratracheally with bleomycin and the vehicle developed severe lung fibrosis, as seen in FIG. 4B. The alveoli have been largely replaced by fibroblasts and extracellular matr...
example f1
Coated Tablet Containing 75 mg of Active Substance
[1524]
[1525] Preparation (Direct Compression)
[1526] The active substance is mixed with all components, sieved and compressed in a tablet-making machine to form tablets of the desired shape.
Weight of core:230 mgAppearance of core:9 mm, biconvex
[1527] The tablet cores thus produced are coated with a film consisting essentially of hydroxypropylmethylcellulose.
Weight of coated tablet:240 mg.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
| Volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com