Perfusion and/or preservation solution for organs
a technology for organs and organs, applied in the field of organ preservation, perfusion and/or reperfusion, can solve the problems of tissue and cellular damage to other organs, affecting the survival of organs, and limiting the success of organ transplantation,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
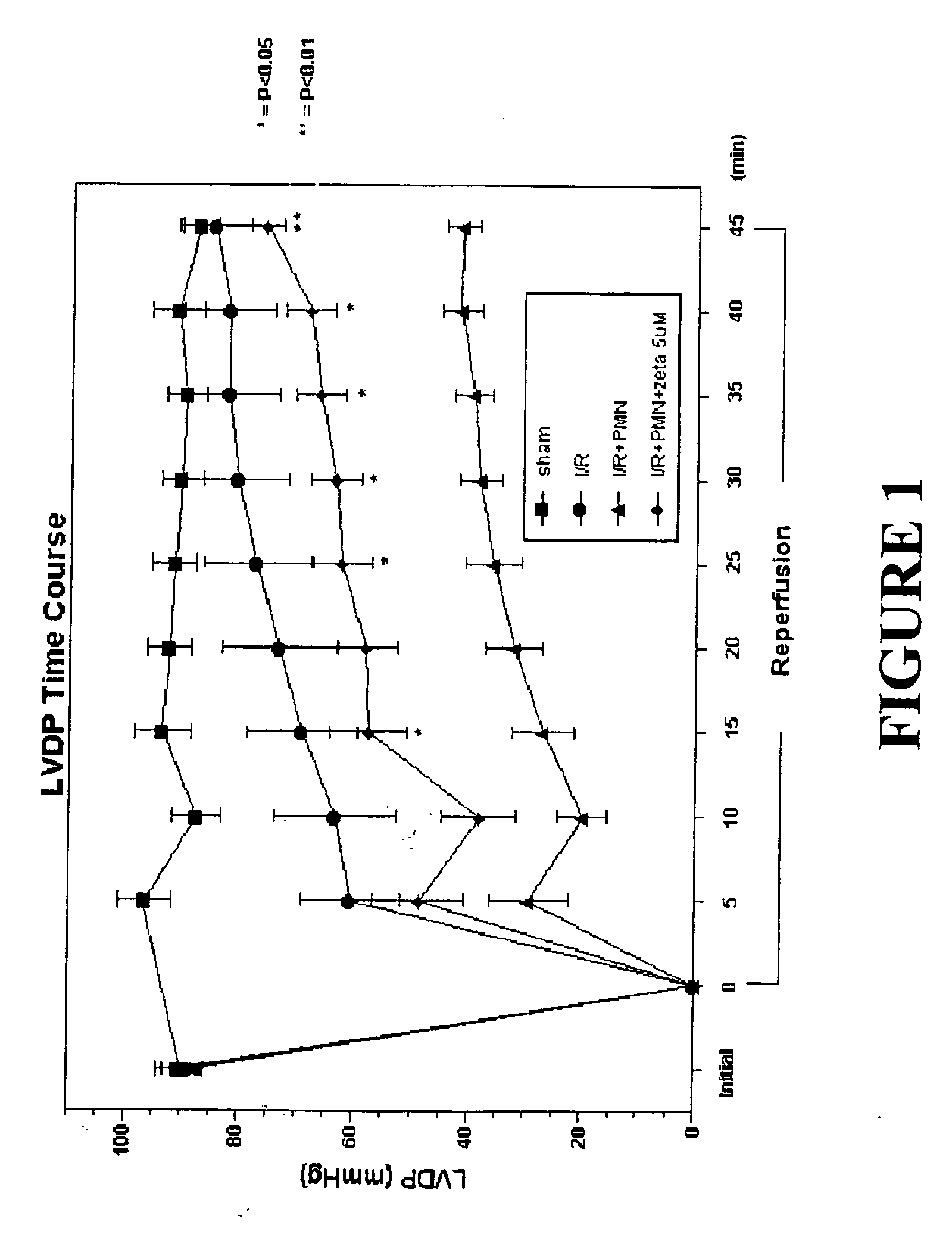
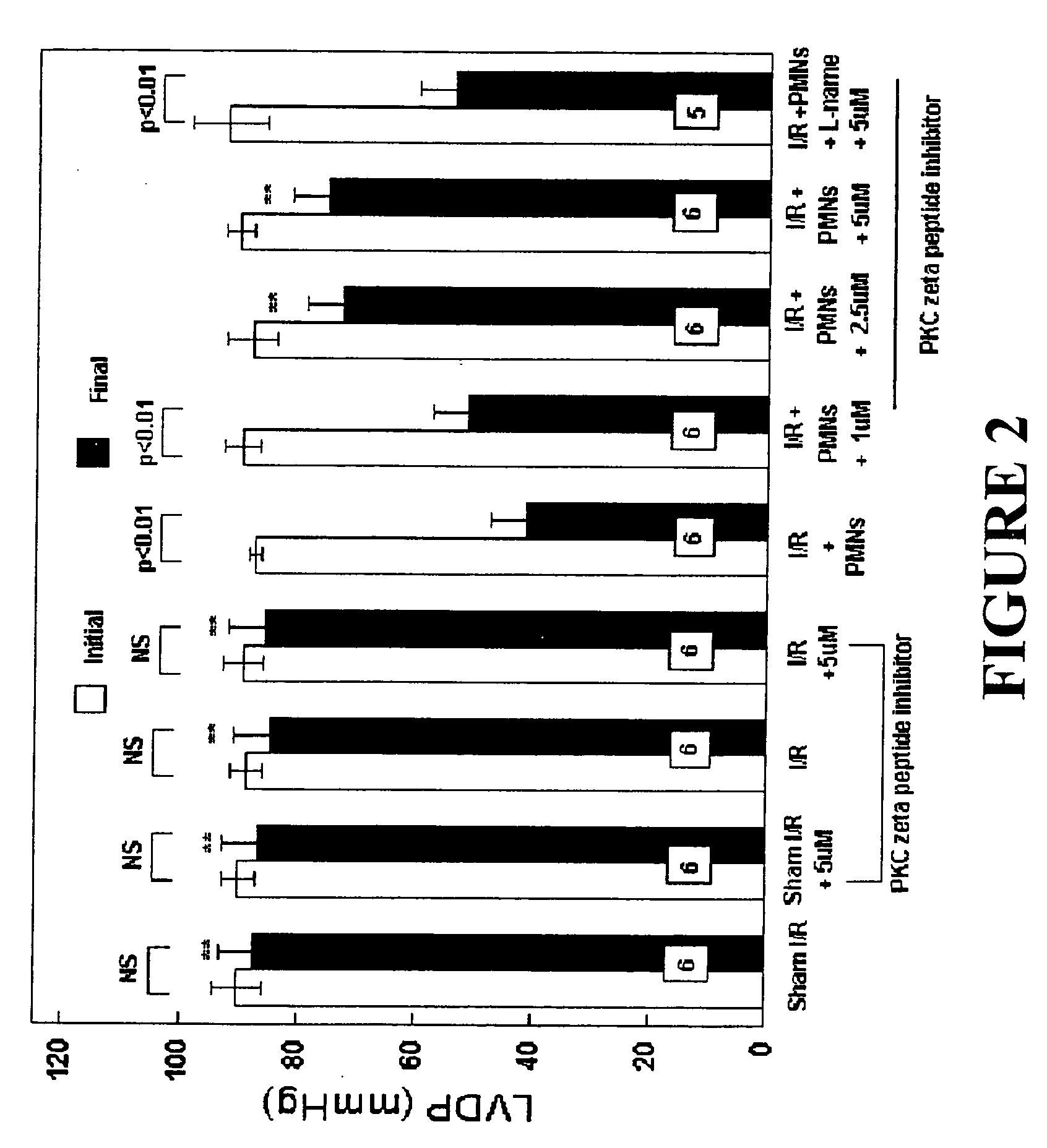
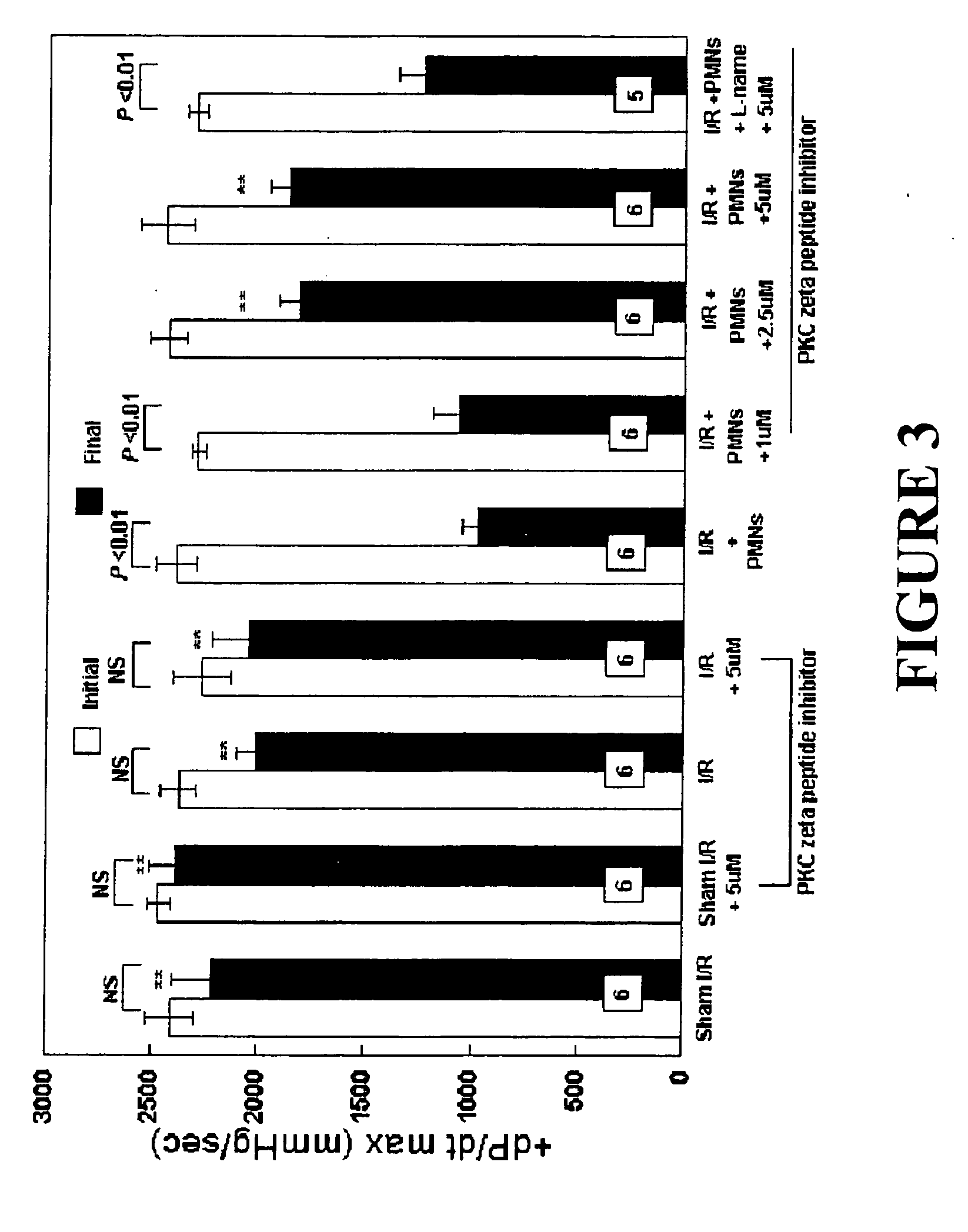
Effects of Peptide Inhibitor of PKC ζ
[0050] Male Sprague Dawley rats (275-325 g, Ace Animals, Boyertown, Pa.) were anesthetized with 60 mg / kg pentobarbital sodium intraperitoneally (i.p.). Sodium heparin (1,000 U) was also administered i.p. The hearts were rapidly excised, the ascending aortas were cannulated, and retrograde perfusion of the heart was initiated with a modified Krebs buffer maintained at 37° C. at a constant pressure of 80 mmHg. The Krebs buffer had the following composition (in mmol / l): 17 dextrose, 120 NaCl, 25 NaHCO3, 2.5 CaCl2, 0.5 EDTA, 5.9 KCl, and 1.2 MgCl2. The perfusate was aerated with 95% O2 and 5% CO2 and equilibrated at a pH of 7.3-7.4. The two side arms in the perfusion line proximal to the heart inflow cannula allowed PMNs, plasma without PKC ζ peptide inhibitor (control hearts) or plasma containing different concentrations of PKC ζ peptide inhibitor (1, 2.5 or 5 μM) to be directly infused into the coronary inflow line. Coronary flow was monitored by a...
example 2
Effects of Peptide Inhibitor of PKC βII
[0069] Experiments with PKCβ II peptide inhibitors were performed substantially as described in Example 1 for PKCζ peptide inhibitors.
[0070] The following groups of isolated perfused rat hearts were used:
[0071] Group 1: Sham I / R hearts were not subjected to ischemia and were not perfused with PMNs, but were perfused with 5 mL of plasma (1 mL / min) at 35 minutes into perfusion (the same time point that I / R hearts would be given 5 mL of plasma, 15 minutes of baseline recordings plus 20 minutes ischemia). These hearts represented a control group to determine if the isolated rat heart can maintain LVDP and +dP / dtmax throughout the 80-minute protocol (n=6).
[0072] Group 2: Sham I / R+PKC βII peptide inhibitor (10 μM) hearts were not subjected to ischemia and not perfused with PMNs. These hearts were administered the PKC βII peptide inhibitor (10 μM, dissolved in plasma from a 5 mM stock in H2O) 35 minutes into perfusion. This group was employed to d...
example 3
Effects of Peptide Inhibitors of PKC βII and PKC ζ in Combination
[0089] Experiments with PKC βII and PKC ζ peptide inhibitors were performed substantially as described in Examples 1 and 2.
[0090] The following groups of isolated perfused rat hearts were used:
[0091] Group 1: Sham I / R hearts were not subjected to ischemia and were not perfused with PMNs, but were perfused with 5 mL of plasma (1 mL / min) at 35 minutes into perfusion (the same time point that I / R hearts would be given 5 mL of plasma, 15 minutes of baseline recordings plus 20 minutes ischemia). These hearts represented a control group to determine if the isolated rat heart can maintain LVDP and +dP / dtmax throughout the 80-minute protocol (n=6).
[0092] Group 2: Sham I / R+PKC βII (10 μM)+PKC ζ (5 μM) peptide inhibitors hearts were not subjected to ischemia and not perfused with PMNs. These hearts were administered the PKC βII and PKC ζ peptide inhibitor (10 μM and 5 μM, respectively, dissolved in plasma from a 5 mM stock i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com