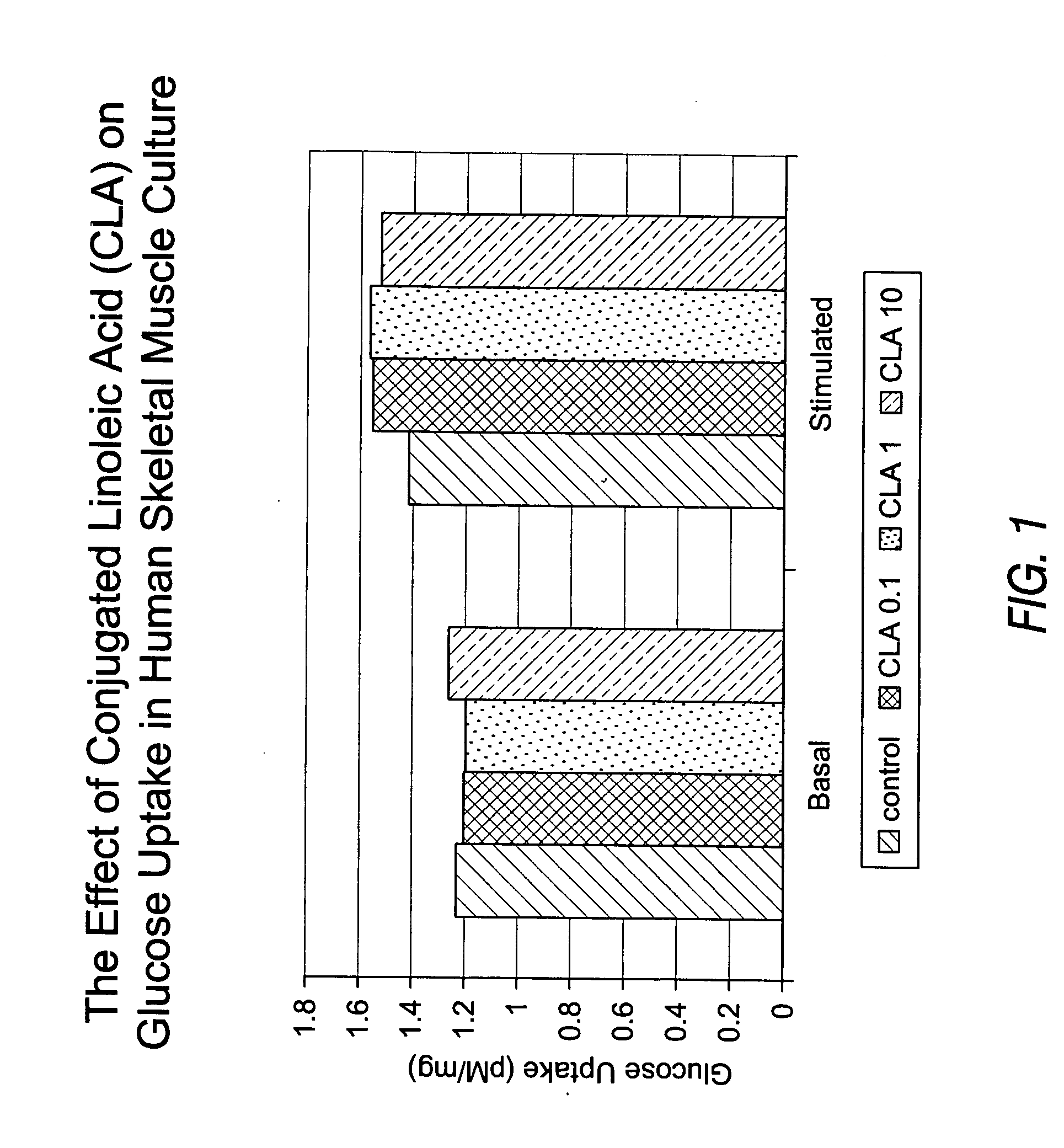
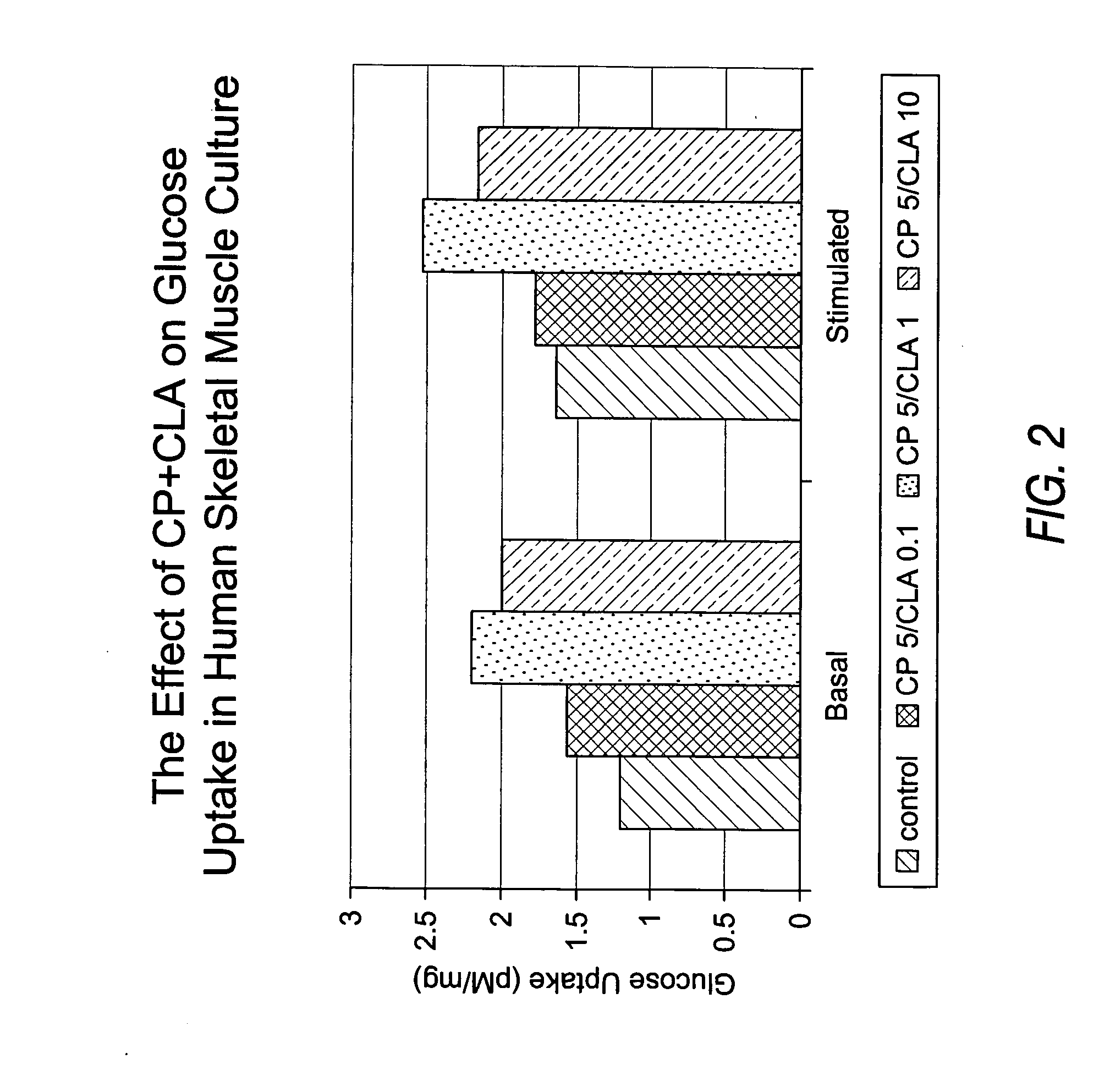
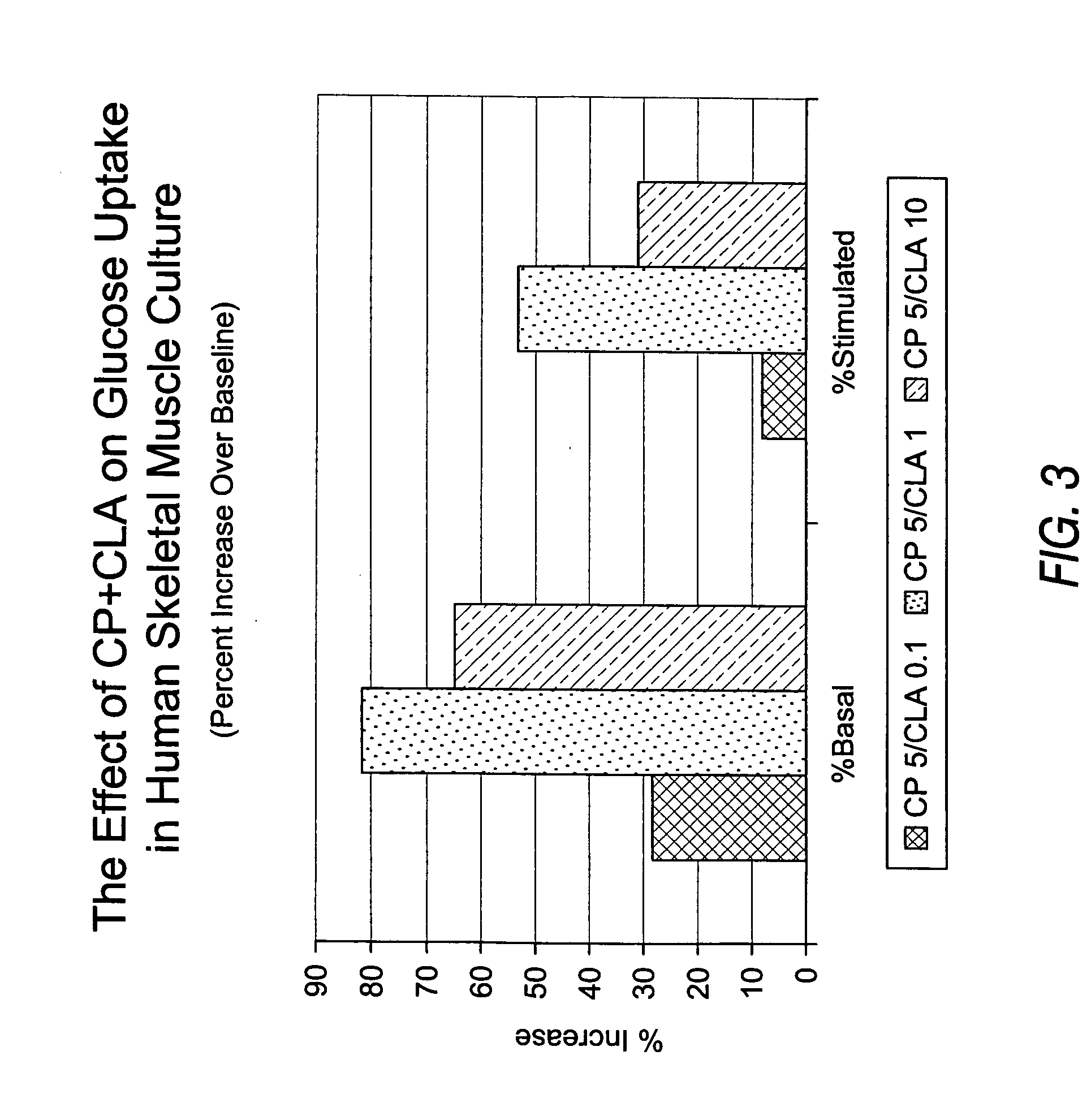
Methods for the treatment of diabetes, the reduction of body fat, improvement of insulin sensitivity, reduction of hyperglycemia, and reduction of hypercholesterolemia with chromium complexes, conjugated fatty acids, and/or conjugated fatty alcohols
a type 1 diabetes and chromium complex technology, applied in the direction of drug compositions, plant/algae/fungi/lichens ingredients, drug compositions, etc., can solve the problems of long-term treatment costs associated with diabetic care, obesity is associated with high cholesterol, and damage to every major organ system in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0055] An obese adult human subject is identified. The subject is orally administered a tablet containing about 300 μg chromium as chromium picolinate and 1 gram conjugated linoleic acid twice a day. The tablet additionally comprises ibuprofen in a pharmaceutically effective dose of 200 mg. Over the course of several weeks, a decrease in body mass is observed. The chromium picolinate in combination with conjugated linoleic acid synergistically reduce the subject's body mass.
[0056] It will be appreciated that although specific embodiments of the invention have been described herein for the purposes of illustration, various modifications may be made without deviating from the spirit and scope of the invention. Accordingly, the invention is not limited except as by the appended claims.
example 2
[0057] A subject suffering from insulin-dependent diabetes is identified. The subject is orally administered a daily dose of one tablet containing about 500 μg chromium as chromium nicotinate and 500 mg conjugated linoleic acid. Over the course of several days, an improvement in glucose uptake in the subject is observed and insulin dependence is reduced. The chromium nicotinate in combination with linoleic acid act synergistically to improve the subject's glucose tolerance and to treat the subject's diabetes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy | aaaaa | aaaaa |
| Insulin resistance | aaaaa | aaaaa |
| weight losses | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com